Artificial Sweeteners in Sugar-Free Ice Cream
In this post, I'll be looking at the artificial sweeteners that are used in sugar-free ice cream production. These will include aspartame, neotame, saccharin, acesulfame potassium, sucralose, and, cyclamate. For each sweetener, I'll cover the acceptable daily intake (ADI), sweetness relative to sucrose (table sugar), use in cooking, metabolism, and health concerns. This will be the first of four posts on sugar-free ice cream production. Part 2 will cover Natural Sweeteners, Part 3 Bulk Sweeteners, and Part 4 Sugar-Free Ice Cream Formulations.
YOU MIGHT ALSO FIND THE FOLLOWING POSTS USEFUL
- Cuisinart ICE-100 Ice Cream and Gelato Maker - Review
- How to make Homemade Vanilla Ice Cream
- Sugar in Ice Cream
- Ice crystal formation and growth in ice cream
1. ICE CREAM COMPOSITION
Ice cream generally contains seven categories of ingredients: fat (dairy or nondairy), milk solids-not-fat (the lactose, proteins, minerals, water-soluble vitamins, enzymes, and some minor constituents), sweeteners, stabilisers, emulsifiers, water, and flavours (Goff & Hartel, 2013). Sucrose (table sugar) has traditionally been the most frequently used sweetener in ice cream production, with a combination of sucrose (10-12%) and corn starch hydrolysate syrup (CSS) (3-5%) now being the most common choice of sweetener (Goff & Hartel, 2013).
For people with diabetes, however, the large amount of sucrose normally used in ice cream needs to be replaced with an acceptable sweetener to control blood glucose.
2. THE GLYCEMIC INDEX
The glycemic index (GI) is a measure of the ability of the carbohydrate in food to raise blood sugar (glucose) levels after consumption compared with an equivalent dose of glucose (Whelan et al. 2008). The reference value is the increase in blood sugar after the intake of 50g of glucose (GI = 100%). The GI of maltose (105) is higher, but the GI of sucrose (65), lactose (46), and fructose (23) is lower (Belitz et al. 2009). Low GI foods release glucose slowly into the blood, producing a gradual and relatively low rise in blood glucose and insulin levels (Wheelan et al., 2008).
3. TYPE OF SWEETENERS
Based on their relative sweetness compared to sucrose, sweeteners are divided into two classes. Sweeteners that, owing to their intense sweetness, produce the required sweetness in small quantities, are called ‘intense’ sweeteners. The other class of sweeteners, known as 'bulk' sweeteners, comprises substances with sweetness a little less than or comparable to that of sucrose. I'll be covering 'bulk' sweeteners in a separate post.
3.1. INTENSE SWEETENERS
Six high-intensity, or nonnutritive, artificial sweeteners are currently approved by the USFDA as food additives. These are aspartame, neotame, advantame, saccharin, acesulfame potassium, and sucralose. Cyclamate is permitted as a food additive in the EU, Canada, Central and South America, and Asia, but, as of September 2016, is not currently approved for use in the U.S. due to health concerns (Price et al., 1970). Several naturally occurring sweet substances, including thaumatin and stevia, are also approved for use as food additives in the EU but but not in the U.S.
In 2011, the global market share for intense sweeteners was estimated as follows: aspartame (27.9%), sucralose (27.9%), cyclamate (15,7%), saccharin (13.1%) stevia (8.7%), acesulfame-K (5.2%), and neotame (1.4%) (Leatherhead Food Research, 2011).
3.1.1. ASPARTAME
Aspartame was discovered in 1965 by the chemist James Schlatter who was attempting to develop an anti-ulcer medicine. It is the methyl ester of two amino acids, L-aspartic acid and L-phenylalanine, both of which occur naturally in foods such as meat, milk, fruits, and vegetables (Kroger et al., 2006). Aspartame itself, however, does not occur naturally. It is the most widely used intense sweetener in the U.S, with a 40% share of the U.S high intensity sweetener market (Haley, 2012). It is sold under the brand names Equal, NutraSweet, and Natra Taste.
3.1.1.1. SWEETNESS RELATIVE TO SUCROSE
Aspartame is 160-220 times as sweet as sucrose (Gougeon et al., 2004). It has a clean, sweet flavour profile that is quite similar to that of sucrose (DuBois, 2000), does not have a bitter aftertaste, and has been found to act as a flavour enhancer, particularly of orange, lemon, grapefruit, and strawberry flavours (Bahoshy et al., 1977; Wiseman & McDaniel, 1991). In a study on the effects of artificial sweeteners on food intake and satiety, aspartame was found to have a more pleasant taste compared with stevia or sucrose (Anton et al, 2010).
3.1.1.2. ACCEPTABLE DAILY INTAKE
The acceptable daily intake (ADI) is defined as the estimated amount (usually expressed in milligrams per kilogram of body weight per day) that a person can safely consume on average every day over a lifetime without risk (ADA, 2004). The ADI is usually set at 1/100 of the maximum level at which no adverse effect was observed in animal experiments (Kroger et al., 2006).
ADI values used in the United States are established by the U.S. Food and Drug Administration (USFDA). International bodies, including the European Union’s Scientific Committee on Food (SCF) and the Joint Expert Committee on Food Additives (JECFA) of the United Nations’ World Health Organisation and Food and Agriculture Organisation, also set ADIs for food ingredients.
According to the USFDA, the ADI of aspartame for humans is 50 mg/kg body weight for both adults and children, while the JECFA has set this value as 40 mg/kg of body weight per day.
3.1.1.3. METABOLISM
Aspartame is metabolised in the small intestine. Enzymes in the digestive track break it down into its components, yielding about 50% phenylalanine, 40% aspartic acid, and 10% methanol alcohol by weight (Roberts, 1988), each of which is then metabolised just as it would be if derived from other dietary sources.
The metabolism of aspartame provides approximately 4 kcal/g of energy, the same calorific value as protein or sucrose. However, the energy added to the diet is negligible, as comparatively little is needed to achieve sweetness comparable to sucrose (Gougeon et al., 2004).
3.1.1.4. USE IN COOKING
Of all sweeteners, aspartame is the most vulnerable to heat (Bell & Labuza, 1991): at high temperatures, it undergoes hydrolysis and loss of sweetness. It is most vulnerable when heated for an extended period of time in a high moisture system at a pH greater than 6 (bell & Labuza, 1991; Wetzel & Bell, 1998). At ice cream pasteurisation temperatures, however, aspartame does not undergo hydrolysis and loss of sweetness.
3.1.1.5. HEALTH CONCERNS
Aspartame has been the subject of much debate with respect to its health effects. It has been described as the most controversial artificial sweetener because of its potential toxicity and mixed reports about its safety (Whitehouse, Boullata, & McCauley, 2008). These include studies linking aspartame consumption to seizures and hyperactivity, brain tumours, headaches, and cancer.
3.1.1.5.1. NEUROLOGICAL AND BEHAVIOURAL PROBLEMS
It has been proposed that aspartame can promote a variety of neurological and behavioural problems, such as seizures and hyperactivity, by increasing phenylalanine levels and/or aspartic acid in the blood (Maher & Wurtman, 1987; Leon et al., 1989; Butchko & Stargel, 2001). However, human studies have demonstrated that aspartame consumption 1.5 times the ADI (Leon et al., 1989) and doses of 2 to 100 mg/kg (Filer & Staging, 1988; Lieberman et al., 1988; Stokes et al., 1991) does not raise blood concentrations of the 3 components to levels associated with adverse effects.
Controlled studies have found no evidence of any neurologic or behavioural effects of aspartame in healthy adults or children (Lapierre et al., 1990; Garriga et al., 1991; Wolraich et al., 1994; Spiers et al., 1998), no effect of aspartame on cognition or behaviour in children with attention deficit disorder (Shaywitz et al 1994a), and no association between aspartame and seizures in individuals with seizure disorders (Shaywitz et al., 1994b; Rowan et al, 1995).
3.1.1.5.2. HEADACHES
Several studies have reported aspartame to be a trigger in causing headaches, with some people being more susceptible to this malady (Van Den Eeden et al. 1994; Lipton et al., 1989; Newman & Lipton, 2001). Blumenthal (1997) reported three case studies wherein women aged 40, 32, and 26 all experienced migraines while chewing a popular gum with aspartame. In all cases, the migraines were relived after cessation of product use. The headaches were reproducible by reintroducing the gum.
3.1.1.5.3. PHENYLKETONURIA
Because it contains phenylalanine, the consumption of aspartame by persons with the rare hereditary disease phenylketonuria (PKU) is generally discouraged by healthcare professionals, although persons with PKU have been shown to tolerate small amounts of aspartame (< 45 mg/kg/day) (Trefz et al., 1994; Mackey & Berin, 1992). Individuals who suffer from PKU lack or have insufficient amounts of the enzyme phenylalanine hydroxyls required to breakdown phenylalanine. Without the presence of this enzyme, phenylalanine accumulates and its buildup can significantly alter human brain function.
3.1.1.5.4. CANCER
There are conflicting results from studies on the genotoxicity of aspartame. Olney et al. (1996) published a report alleging a connection between aspartame and increases in the incidence of brain tumours in humans. However, a subsequent study investigating the relationship between aspartame consumption and brain tumours in children did not find a relationship between aspartame use and an increased risk of brain tumours (Gurney et al., 1997).
Research by Soffritti et al. (2007), using rats, demonstrated a significant increase of malignant tumours in males, an increase in the incidence of lymphomas and leukemias in males and females, and an increase in the incidence of mammary cancer in females. However, several studies on the toxicology of aspartame in rats have demonstrated that aspartame is not genotoxic (Kotsonis and Hjelle, 1996; Jeffrey & Williams, 2000; Durnev et al, 1995; Kulakova et al., 1999; Mukhopadhyay et al., 2000).
BosettIi et al. (2009) examined the association between saccharin and other sweeteners (including aspartame) and gastric, pancreatic, and endometrial cancers in Italy between 1991 and 2004. The results of this study indicate that consumption of sweetener products such as saccharin and aspartame are not associated with gastric, pancreatic, and endometrial cancers among the people studied.
In a review on the health effects of aspartame, Walton (2012) found that 92% of independently funded studies reported that aspartame can cause adverse health effects. Yilmaz & Ucar (2014) reviewed a total of 24 studies on the genotoxicity of aspartame. Based on nearly 55% positive results, they concluded that aspartame is a moderate genotoxic agent. In human studies, they found that 45% yielded positive results, with brain tumour, NHL, leukemia, urinary tract tumours, and multiple myeloma reported in three studies. This lead them to conclude that long-term exposure can play an important role in the development of aspartame induced cancer.
3.1.1.5.5. SUMMARY
Several reevaluations have been performed by risk assessment authorities including the USFDA and the European Food Safety Authority (EFSA), which have deemed aspartame to be of no safety concern at intakes below the specified ADI levels (EFSA 2013; FDA 2014). Following a thorough review of evidence provided both by animal and human studies, the EFSA ruled out a potential risk of aspartame causing damage to genes and inducing cancer. Its experts concluded that aspartame does not harm the brain, nervous system, or affect behaviour or cognitive function in children or adults.
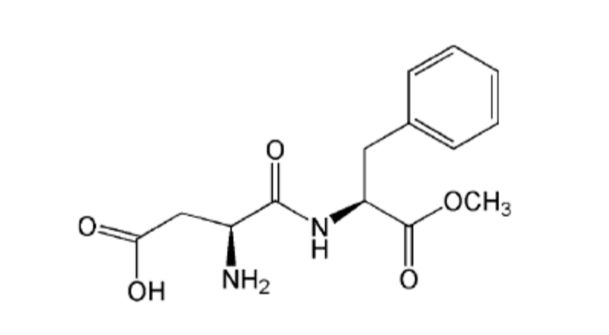
3.1.2. NEOTAME
Neotame, discovered by Nofre & Tinti (1996), is a non-nutritive, non-calorie sweetener composed of two amino acids, L-aspartic acid and L-phenylalanine, and an additional 3, 3-dimethylbutyl group (Kumari et al., 2016a). It is a derivative of aspartame, being made up of 75% aspartame, and was approved by the FDA in 2002 (USFDA 2002) and by the European Union in 2009 (CD, 2009). 3.1.2.1. SWEETNESS RELATIVE TO SUCROSENeotame is one of the sweetest commercially available sweeteners, with a sweetness potency 7,000 to 13,000 times greater than sucrose and 30-60 times greater than aspartame (EFSA, 2007). Because it is extremely sweet, the amount needed to sweeten a food or beverage is extremely small. It has a very clean taste that is close to sucrose, with no undesirable metallic or bitter aftertaste, and a sweetness that develops gradually like sucrose.Like aspartame, neotame intensifies certain flavours, particularly acidic fruit flavours (orange, lemon, and grapefruit) and cherry flavour (Kumari et al., 2016a).
3.1.2.1. SWEETNESS RELATIVE TO SUCROSENeotame is one of the sweetest commercially available sweeteners, with a sweetness potency 7,000 to 13,000 times greater than sucrose and 30-60 times greater than aspartame (EFSA, 2007). Because it is extremely sweet, the amount needed to sweeten a food or beverage is extremely small. It has a very clean taste that is close to sucrose, with no undesirable metallic or bitter aftertaste, and a sweetness that develops gradually like sucrose.Like aspartame, neotame intensifies certain flavours, particularly acidic fruit flavours (orange, lemon, and grapefruit) and cherry flavour (Kumari et al., 2016a).
3.1.2.2. ACCEPTABLE DAILY INTAKE
The ADI for neotame in the United States is 0.3 mg/kg of body weight/d (FDA, 2014). The JFECA established an ADI of 2 mg/kg of body weight/d (JFECFA, 2004).3.1.2.3. METABOLISMApproximately 20% to 30% of ingested neotame is absorbed from the digestive tract. Practically all of both absorbed and unabsorbed neotame is converted into de-esterified neotame, the major metabolite, and an insignificant amount of methanol, both of which are rapidly excreted from the body either in the faeces or the urine (Korger et al., 2006). The effective caloric value of neotame is less than 1.2 kJ/g (<0.3 kcal/g) (Nofre & Tinti, 2000).3.1.2.4. USE IN COOKINGNeotame is heat-stable and thus can be used in cooking and baking: it is stable at 80°C in pH range of 3-5.5, implying that high-temperature-short-time (HTST) processing is possible with ice cream mixes sweetened with neotame (Nofre & Tinti, 2000).Kumari et al., (2016) found that pasteurisation of an ice cream mix at 68°C for 30 minutes resulted in no loss of neotame. However, they found that the amount of neotame decreased significantly from 99.42 to 89.93% during 90 days of storage at -18°C. Therefore, neotame may not be the most suitable sweetener for ice cream intended to be stored for long periods of time (i.e. for sale in a supermarket) because of the resulting loss of sweetness.Neotame itself is stable under dry storage conditions, under which it has an estimated shel life of several years at ambient temperatures (Nofre & Tinti, 2000). Like aspartame, neotame is relatively stable at pH from 3 to 5.5. Unlike aspartame, however, it is compatible with reducing sugars (such as glucose, fructose, high-fructose corn syrup, lactose, maltose) and aldehyde-based flavouring agents (such as vanilla, cinnamon, cherry, bitter almond, lemon) (Nofre & Tinti, 2000).3.1.2.5. HEALTH CONCERNSStudies on various species, including mice, rats, dogs, rabbits, and humans, have shown that neotame is not carcinogenic, teratogenic, or mutagenic, and does not produce any reproductive or developmental toxicity (Aguilar et al., 2007).One of the benefits of neotame over aspartame is the insignificant release of methanol and phenylalanine once metabolised, with no possible hazard for phenylketonuric subjects (Nofre & Tinti, 2000). It is considered safe for all segments of the population, including diabetics at dose levels up to 1.5 mg/kg bw/day (Aguilar et al., 2007).
3.1.3. ADVANTAME
Advantame is an ultrahigh-intensity non-caloric derivative of aspartame that is similar in structure to neotame. It was approved for human consumption by the USFDA in 2014, making it the newest artificial sweetener. As of September 2016, it is yet to be approved for human consumption in the EU.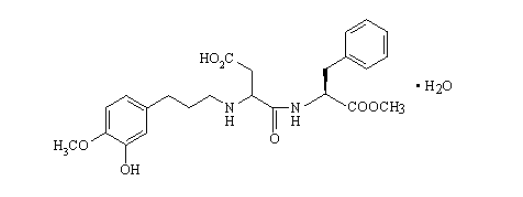
3.1.3.1. SWEETNESS RELATIVE TO SUCROSE
Neotame is the sweetest commercially available artificial sweetener, with a sweetness potency approximately 20,000 times sweeter than sucrose and 200 times sweeter than aspartame (Bishay & Bursey, 2012). This means that a very low concentration is needed to sweeten foods. It has a similar sensory profile to aspartame, especially at high concentrations. It has a dominant sweet flavour, while perceived intensities for bitter and sour flavours are very weak.3.1.3.2. ACCEPTABLE DAILY INTAKEThe ADI of advantame is 32.8 mg/per kg body weight/d (USFDA, 2014).3.1.3.3. METABOLISMAdsorption of advantame and its metabolites occurs almost entirely in the small intestine. It is rapidly but poorly absorbed and the main excretion route is via faeces. The amount absorbed can approach 15% in humans (USFDA, 2014).3.1.3.4. USE IN COOKINGAdvantame is heat stable, meaning that it will stay sweet even when used at high temperatures. Stability studies show that the degradation of advantame is pH, time, and temperature-dependent, and is more likely to occur from its use in low pH (acidic) foods during extended storage conditions. Its use as a sweetener in ice cream may, therefore, give rise to the issue found in ice cream made with neotame: that is, loss of sweetness during extended periods of storage.
3.1.3.5. HEALTH CONCERNS
In determining the safety of advantame, the USFDA reviewed data from 37 animal and human studies designed to identify possible toxic effects, including effects on the immune system, reproductive and developmental systems, and nervous system. The USFDA also reviewed pharmacokinetic and carcinogenicity studies, as well as several additional exploratory and screening studies. Its conclusion was that there is a reasonable certainty that advantame is not harmful.
Advantame is also a suitable sweetener alternative for diabetics. In a review of a clinical study of diabetic subjects designed to investigate the tolerability of repeated daily consumption of a 30 mg dose (equivalent to 0.375 mg/kg bw/day to 0.5 mg/kg bw/day) of advantame fed daily for 12 weeks, the USFDA (2014) concluded that advantame was tolerated at daily doses up to 0.5 mg/kg bw/day in people with type 2 diabetes.
3.1.4. SACCHARIN
Saccharin, the oldest low-calorie sweetener, was discovered in 1879 by Constantine Fahlberg at Johns Hopkins University. It is the least expensive of the of the low-calorie sweeteners and is sold under the brand names Sweet'N Low, Sugar Twin, and Hermesetas.
3.1.4.1. SWEETNESS RELATIVE TO SUCROSE
Saccharin is 200-700 times sweeter than sucrose (Kroger et al., 2006; Shankar, Ahuja, & Sriram, 2013). It has a lingering bitterness (Larson-Powers & Pangborn, 1978), which is more pronounced at higher concentrations and which some people detect more than others do (DuBois, 2000). It is therefore often used in sweetener blends to produce a more sugar-like taste.
3.1.4.2. ACCEPTABLE DAILY INTAKE
The ADI of saccharin for humans is 0-5 mg/kg of body weight (JECFA, UNWHO). It is not recommended during pregnancy (Gougeon et al., 2004).
3.1.4.3. METABOLISM
Saccharin is absorbed, but not metabolised, by the body and is excreted in the urine (Renwick, 1986). It does not affect blood insulin levels (Mukherjee & Sarkar, 2011), making it a viable sugar substitute for people with diabetes.
3.1.4.4. USE IN COOKING
An important characteristic of saccharin is that its sweetening power is not reduced when heated, making it an excellent sweetener in low-calorie and sugar-free products (Shankar, Ahuja, & Sriram, 2013).
3.1.4.5. SAFETY CONCERNS
3.1.4.5.1. BLADDER CANCER
Significant concerns were raised in the 1970s and 80s about the safety of this sweetener. These were based on studies on rats that clearly demonstrated an increased incidence of bladder cancer when rats were fed high amounts of sodium saccharin in their diet from birth (Tisdel et al., 1974; Taylor & Friedman, 1974; Arnold et al., 1980; Schoenig et al., 1985).
Extensive research since these earlier studies suggests that the mechanism by which saccharin causes bladder cancer is specific to rats and does not occur in humans because of differences in bladder physiology and urine chemistry between the 2 species. Furthermore, tumours in rats occur only after a lifetime exposure to very high doses of saccharin, equivalent to the human consumption of hundreds of servings of saccharin-sweetened foods or beverages per day, every day for a life-time (Cohen et al., 1998, Ellwein & Cohen, 1990).
In a epidemiological study in China, Yu et al. (1997) reported that frequent saccharin use almost quadrupled bladder cancer risk in humans. A number of studies examining the association between saccharin intake and bladder cancer in human populations, however, do not support a relationship between lower urinary tract cancer and the consumption of saccharin (Armstrong et al., 1976; Wynder & Goldsmith, 1977; Morrison & Buring, 1980; Walker et al, 1982; Morgan & Wong, 1985; Elcock & Morgan,1993; Gallus et al., 2007).
3.1.4.5.2. SUMMARY
On the basis of its carcinogenicity in rats, sodium saccharin was listed as a potential human carcinogen (Arnold et al., 1983; Merrill & Taylor, 1985). In 1999, the International Agency for Research on Cancer (IARC) removed saccharin from its list of substances ‘probably carcinogenic to humans’, noting that the ability of saccharin to cause bladder tumours in rats ‘appears to be unique to the rat… …not relevant to humans because of critical interspecies difference sin urine composition’. Similarly, in 2000, the National Toxicology Program removed saccharin from its official list of substances known or reasonably anticipated to be human carcinogens (NTP, 2000).
3.1.5. ACESULFAME POTASSIUM
Acesulfame potassium (acesulfame-K) was discovered in Germany in 1967 by the chemist Karl Clauss. It is an organic salt, containing sulfur and nitrogen, and is sold under the brand names Sunette, Sweet One, and Swiss Sweet.
3.1.5.1. SWEETNESS RELATIVE TO SUCROSE
Acesulfame potassium is 150-200 times sweeter than sucrose (Whitehouse, 2008).
3.1.5.2. ACCEPTABLE DAILY INTAKE
The ADI for acesulfame-K is 15 mg/kg body weight (JECFA, UNWHO).
3.1.5.3. METABOLISM
Acesulfame-K is absorbed by the gut and excreted in the urine without being metabolised and is therefore not a source of energy (Renwick, 1986).
3.1.5.4. USE IN COOKING
Because acesulfame-K is heat-stable, it can be used in cooking and baking (Nabors, 2002). When used alone in quantities needed to achieve adequate sweetness, it leaves a bitter aftertaste (Mortensen, 2006). It is therefore often combined with other intense sweeteners, usually aspartame or sucralose, to intensify its sweetness and decrease its bitter taste (Duffy & Anderson, 1998). Such combinations provide a more sugar-like profile (Meyer & Riha, 2002) and also decrease the total amount of sweetener used.
3.1.5.5. HEALTH CONCERNS
3.1.5.5.1. CANCER
Mukherjee & Chakrabarti (1997) found that when the dosage of acesulfame-K administered to mice was within the ADI of 15 mg/kg of body weight, the number of chromosomal aberrations was not significant compared to control mice. However, at higher doses (60, 450, 1,100, and 2,250 mg/kg), acesufame-K was blastogenic and genotoxic. Whitehouse et al. (2008) note that these results demonstrate unequivocally that acesulfame-K interacts with DNA to produce genetic damage, but doses capable of producing damage are well above the ADI.
In its reevaluation of acesllfame-K , the SCF assessed the study conducted by Mukherjee & Chakrabarti and concluded that its adequacy was questionable. The SCF noted that when a second group of researchers attempted to replicate the results of the study, they were unable to do so (SCF, 2000). In a follow-up study to Mukherjee & Chakrabarti (1997), Mukhopadhyay et al. (2000) found no synergistic genotoxic effects when acesulfame-K was used in combination with aspartame in mice.
3.1.5.5.2. ACETOACETAMIDE
One of the byproducts of acesulfame-K’s breakdown in the body is acetoacetamide, which is toxic at high doses (Shankar, Ahuja, & Sriram, 2013). However, the typical amounts of acesulfame-K consumed by the average population are quite low that toxicity is highly unlikely (Shankar et al., 2013). Lino et al (2008) found that the amount of acesulfame-K and aspartame consumed in soft drinks by Portuguese teenagers was well below the ADI and that this population was noted to be at low risk from any adverse effects arising from the use of these artificial sweeteners. In the United States, actual consumption of acesulfame-K is about 20% of the ADI over a lifetime (IFIC Review, 2009).
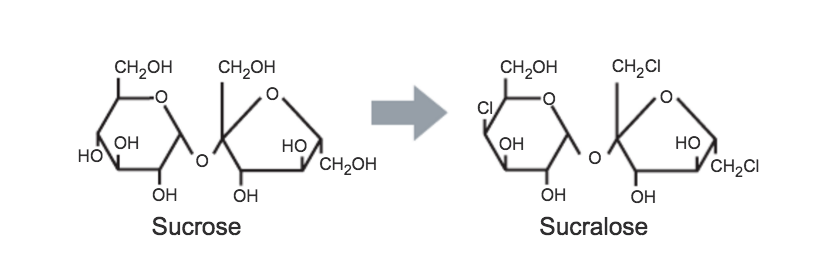
3.1.6. SUCRALOSE
Sucralose was discovered by British researchers in 1976. It is derived from sucrose by the selective replacement of three hydroxyl groups by chlorine atoms and is sold under the brand name Splenda.
3.1.6.1. SWEETNESS RELATIVE TO SUCROSE
Sucralose is about 600 times sweeter than sucrose (Gougeon et al., 2004). In a study comparing the sensory profile of several sweeteners, it was found that sucralose is most similar to sucrose (Cardoso & Blini, 2008), but unlike sucrose, sucralose does not promote tooth decay (Mandel & Grotz, 2002). It also does not leave an unpleasant or bitter aftertaste.
3.1.6.2. ACCEPTABLE DAILY INTAKE
The ADI for sucralose in the United States is 5 mg/kg body weight per day (USFDA), and 0-15 mg/kg body weight in the European Union (JECFA, UNWHO).
3.1.6.3. METABOLISM
Sucralose is not hydrolysed in the intestine and less than 25% of an oral dose is absorbed (Gougeon et al., 2004). The small proportion that is absorbed is not metabolised and is excreted unchanged in the urine (Roberts et al., 2000). Therefore, sucralose doesn't add any calories.
3.1.6.4. USE IN COOKING
Sucralose is exceptionally stable and is able to retain its sweetness when subjected to high heat and acidic treatment (Knight, 1994). Commercially, it is combined with maltodextrin, which enables it to physically replace sucrose (Roberts et al., 2000).
3.1.6.5. HEALTH CONCERNS
3.1.6.5.1. HEADACHES
Case studies have identified sucralose to be a causative agent in triggering migraine headaches (Bigal & Krymchantowski, 2006; Patel, Sarma, & Grimsley, 2006).
3.1.6.5.2. ADVERSE EFFECTS IN THE GI TRACT
Studies investigating the safety of acute and chronic consumption of sucralose at ADI levels have not reported any adverse effects on human (Baird et al., 2000; Grice & Goldsmith, 2000) or animal health (Sims et al., 2000; John, Wood, & Hawkins., 2000a; John, Wood, & Hawkins, 2000b; Wood, John, & Hawkins, 2000; Mann et al., 2000).
However, a controversial study by Abou-Donia et al. (2008) found that sucralose caused adverse effects in the GI tract. In this study, rats that consumed Splenda for 12 weeks had a significant decrease in beneficial gut bacteria with resulting weight gain, increased decal pH due to decreased production of short-chain fatty acids by colonyc bacteria, and enhanced expression of cytochromes in the body that can potentially affect bioavailability of nutrients and drugs. Furthermore, the stated changes in the GI tract occurred when when the rats were fed sucralose at low doses approved by the FDA for human consumption. These results have been, however, widely criticised, with an expert panel report citing that the study is ‘deficient in several critical areas’ and that the conclusions from he study are not ‘scientifically valid’ (Brusick et al., 2009).
3.1.6.5.3. SUMMARY
Sucralose is considered to be safe for long-term use (Brusick et al., 2010), as well as for people with diabetes. A 3 month study of 128 people with diabetes, in which sucralose was administered at a dose approximately 3 times the maximum estimated daily intake, showed no adverse effects on any measure of blood glucose control (Grotz et al., 2003).
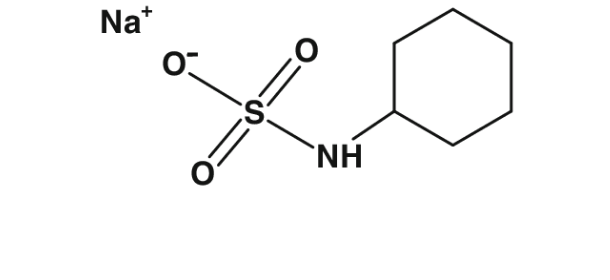
3.1.7. CYCLAMATE
Three different compounds are referred to as cyclamates: cyclamic acid, calcium cyclamate, and sodium cyclamate (Mortensen, 2006).
3.1.7.1. SWEETNESS RELATIVE TO SUCROSE
Cyclamate has the lowest sweetening power of the intense sweeteners. It is only 30-50 times sweeter than sucrose (Gougeon et al., 2004), meaning that relatively large amounts need to be used to sweeten a food or beverage.
3.1.7.1. ACCEPTABLE DAILY INTAKE
In 2000, the SCF established a full ADI of 0-7mg/kg body weight for cyclamic acid and its calcium and sodium salts (SCF, 2000). Cyclamate is not recommended during pregnancy (Gougeon et al., 2004).
3.1.7.2. HEALTH ISSUES
3.1.7.2.1. BLADDER TUMOURS
Cyclamate was extensively used during the 1960s, often in a 10:1 blend with saccharin for a better taste than that of either sweetener alone (DuBois, 2000). In 1969, however, it was prohibited in many countries because bladder tumours were found in rats fed with the 10:1 cyclamate-saccarin mixture (Price et al., 1970). Extensive further studies in rats, mice, dogs, hamsters, and monkeys, however, have not shown any link between cyclamate and cancer (Bopp, Sonders, & Kesterson, 1986).
3.1.7.2.2. CYCLOHECYLAMINE
Establishing an ADI for cyclamate is difficult because different people metabolise this sweetener in different ways. Some people excrete all or practically all of it unchanged, while others convert variable amounts - occasionally as much as 85% (SCF 2000) - of ingested cyclamate into a metabolite called cyclohecylamine. High doses of cyclohexylamine have been shown to cause testicular atrophy in rats (Bopp et al., 1986). A study by Serra-Jajem et al. (2003), however, supported the lack of an association between cyclamate and cyclohexylamine and male infertility in humans.
4. SUMMARY
In this post, I've introduced the artificial sweeteners t used in sugar-free ice cream production. For each sweetener, I've looked at the acceptable daily intake (ADI), sweetness relative to sucrose (table sugar), use in cooking, metabolism, and health concerns.
I'd love to hear from you if you have any questions or suggestions on how this post can be improved so do get in touch! All the best, Ruben :)
References:
Abou-Donia, M. B., El-Masry, E. M., Abdel-Rahman, A. A., McLendon, R. E., and Schiffman, S. S., 2008. Splenda alters gut microflora and increases intestinal P-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health. 71:1415-29.
(ADA) American Diatetic Association. 2004. Position of the American Dietetic Assn.: use of nutritive and nonnutritive sweeteners. J Am Diet Assoc. 104:255-75.
Aguilar, F., Autrup, H., Barlow, S., Castel, L., Crebelli, R., Dekant, W., Toldra, F., 2007. Neotame as a sweetener and flavour enhancer. Scientific opinion of the panel on food additives, falvourings, processing aids and materials in contact with food. EFSA J. 581, 1-43.
Ahmed, F.E., and Thomas, D. B., 1992. Assessment of the carcinogenicity of the nonnutritive sweetener cyclamate. Crit Rev Toxicol. 22:81-118.
Anton, S. D., Martin, C. K, Han, H., Coulon, S., Cefalu, W. T., Geiselman, P. et al., 2010. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 55: 37-43.
Armstrong, B., Lea, A. J., Adelstein, A. M., et al. 1976 Cancer mortality and saccharin consumption in diabetics. Br J Prev Soc Med. 30:151-157.
Arnold, D. L., Moodie. C. A., Grice, H. C., Charbonneau, S. M., Stavric, B., Collins, B. T., McGuire, P. F., Zawidzka, Z. Z., and Munro, I. C. 1980. Long-term toxicity of ortho-toluenesulfonamide and sodium saccharin in the rat. Toxicol. Appi. Pharmacol. 52: 113-152.
Arnold, D. L., Krewski. D., and Munro, I. C., 1983. Saccharin: a toxicological and historical perspective. Toxicology. 27: 179-256.
Bahoshy, B. J., Klose, R. E., and Nordstrom, H. A., 1977. Chewing gum of longer lasting sweetness and flavour. 1972. U/S/ Patent No. 4,036,992.
Baird, I. M., Shephard, N. W., Merritt, R. J., et al. 2000. Repeated dose study of sucralose tolerance in human subjects. Food Chem Toxicol. 38(suppl 2):S123-S129.
Belitz, H.D., Grosch, W., & Schieberle, P., 2009. Food Chemistry (4th ed). Berlin: Springer.
Bell, L. N., and Labuza, T. P., 1991. Aspartame degredation kinetics as affected by pH in intermediate and low moisture food systems. Journal of Food Science. 56(1).
Bigal, M. E., and Krymchantowski, A. V., 2006. Migraine triggered by sucralose: A case report. Headache. 46:515-7
Blumenthal, H. J., 1997. Chewing gum headaches. Headache, 37(10).
Bopp, A. B., Sonders, R. C., Kesterson, J. W., 1986. Toxicological aspects of cyclamate and cyclohexylamine. Crit Rev Toxicol. 16: 213306.
BosettIi, C., Gallus, S., Talamini, R., Montella, M., Franceshci, S., Negri, E., et al. 2009. Artificial sweeteners and the risk of gastric, pancreatic, and endometrial cancers in Italy. Cancer Epidemiol Biomarkers Prev. 18:2235-8.
Brusick, D., Borzeleca, J. F., Gallo, M., Williams, G., Kille, J., Hayes, A. W., et al., 2009. Expert panel report on a study of Splenda in male rats. Regul Tozicol Pharm. 55:6-12.
Brusick, D., Grotz, V. L., Slesinski, R., Kruger, C. L., and Hayes, A. W., 2010. The absence of genotoxicity of sucralose. Food Chem Toxicol. 48:3067-72.
Butchko, H. H., Stargel, W. W., 2001. Aspartame: scientific evaluation in the postmarketing period. Regul Toxicol Pharmacol. 34:221-233.
Cardoso, J. M. P., and Bolini, H. M. A., 2008. Descriptive profile of peach nectar sweetened with sucrose and different sweeteners. J Sens Stud. 23:804-16
[CD] Commission Directive 2009/163/EU of 22 December 2009 amending Directive 94/35/EC of the European Parliament and of the Council on sweeteners for use in foodstuffs with regard to neotame
Cohen, S. M., Anderson, T. A., de Oliverira, L. M., and Arnold, L. L., 1998. Tumorigenicity of sodium ascorbate in male rats. Cancer Res. 58:2557-61
Dubois, G. E., 2000 Sweeteners: nonnutritive. In: Francis, F. J., ed. Encyclopaedia of food science and technology. 2nd ed. Vol 4. New York: John Wiley & Sons, Inc.
Durnev, A. D., Oreshchenko, A. V., Kulakova, A. V., Beresten, N. F., and Seredenin, S. B., 1995. Clastogenic activity of dietary sugar substitutes. Vopr. Med. Khim. 41, 31–33.
[EFSA]. 2007. European food safety authority.Neotame as a sweetener and flavour enhancer. The EFSA Journal. 581, 1-43.
EFSA, 2013. Scientific opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA Journal 11: 3496.
Elcock, M., and Morgan, R. W., 1993. Update on artificial sweeteners and bladder cancer. Regul Toxicol Pharmacol. 17(1):35-43.
Ellwein, L. B., and Cohen, S. M., 1990. The health risks of saccharin revisited. Crit. Rev.Toxicol. 20: 311-326.
FDA., 2014. Additional information about high-intensity sweeteners permitted for use in food in the United States. Available at www.fda.gov/Food/IngredientsPackagingLaveling/FoodAdditivesIngredients/ucm397725.html
Filer, L. J., and Stegink, L. D., 1988. Effect of aspartame on plasma phenylalanine concentrations in humans. In R. J. Wurtman & E. Ritter-Walker (Eds.), Dietary phenylalanine and brain function (pp. 18-40). Boston: Birkhauser.
Fry, J., 1999. The world market for intense sweeteners. World Rev Nut Diet. 85:201-11.
Gallus, S., Scotti, L., Negri, E., Talamini, R., Franceschi, S., Montella, M., et al. 2007. Artificial sweeteners and cancer risk in a network of case-control studies. Ann Oncol. 18:40-4.
Goff, H. D., and Hartel R. W., 2013. Ice Cream. Seventh Edition. New York Springer.
Gougeon, R., Spidel, M., Lee, K., Field, C. J., 2004. Canadian Diabetes Association National Nutrition Committee Technical Review: Non-nutritive Intense Sweeteners in Diabetes Management. Canadian Journal of Diabetes. 28(4):385-399.
Garriga, M. M., Berkebile, C., and Metcalfe, D. D., 1991. A combined singleblind, double-blind, placebo-controlled study to determine the reproducibility of hypersensitivity reactions to aspartame. J Allergy Clin Immunol. 87:821-827.
Grice, H. C., and Goldsmith, L. A., 2000. Sucralose—an overview of the toxicity data. Food Chem Toxicol. 38(suppl 2):S1-S6.
Grotz, V. L., Henry, R. R., McGill, J. B., Prince, M. J., Shaman, H., Trout, J. R., and Pi-Sunyer, F. X., 2003. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc. 1003:1607-12.
Gurney, J. G., Pogoda, J. M., Holly, E. A., et al. 1997. Aspartame consumption in relation to childhood brain tumor risk: results from a case-control study. J Natl Cancer Inst. 89:1072-1074.
Haley, S., 2012. Sugar and Sweeteners Outlook. Electronic Outlook Report from the Economic Research Service, United States Department of Agriculture. Available at http://www.ers.usda.gov/media/372138/sssm283.pdf
IFIC Review: Low-Calorie Sweeteners and Health. Food Insight - Your nutrition and food safety resource. 2009. Available atwww.foodinsight.org/resources/detail.aspxtopic=IFIC_Review_Low_Calorie_Sweeteners_and_Health.
Jeffrey, A. M., and Williams, G. M., 2000. Lack of DNA-damaging activity of five non-nutritive sweeteners in the rat hepatocyte/DNA repair assay. Food Chem. Toxicol. 38, 335–338.[JFECFA] Joint FAP/WHO Expert Committee on Food Additives. 2004. Safety evaluation of certain food additives and contaminants. Neotame. WHO Food Aditives Series 52. Available at: www.inchem.org.documents/jecfa/jecmono/v52je08.html
John, B. A.,Wood, S. G., and Hawkins, D. R., 2000(a) The pharmacokinetics and metabolism of sucralose in the rabbit. Food Chem Toxicol. 38(suppl 2):S111-S113.
John, B. A, Wood, S. G., and Hawkins, D. R., 2000(b). The pharmacokinetics and metabolism of sucralose in the mouse. Food Chem Toxicol. 38(suppl 2):S107-S110.
Knight, I., 1994. The development and application of sucralose, a new high-intensity sweetener. Can J Physiol Pharmacol. 72:435-9.
Kotsonis, F. N., and Hjelle, J, J., 1996. The safety assessment of aspartame: Scientific and regulatory considerations. In The Clinical Evaluation of a Food Additive: Assessment of Aspartame (C. Tschanz, H. H. Butchko,W.W. Stargel, and F.N. Kotsonis, Eds.), pp. 23–41. CRC Press, Boca Raton, FL.
Kroger, M., Meister, K., and Kava, R. 2006. Low-calorie sweeteners and other sugar substitutes: A review of the safety issues. Comprehensive Reviews in Food Science and Food Safety. 5(2).
Kumari, A., Choudhary, S., Arora, S., Sharma, V., 2016a. Stability of aspartame and neotame in pasteurized and in-bottlesterilized flavoured milk. Food Chemistry. 196.
Kumari, A., Arora, S., Singg, A. K., Choudhary, S., 2016b. Development of an analytical method for estimation of neotame in cake and ice cream. LWT – Food Science and Technology. 70. 142-147.
Lapierre, K. A., Greenblatt, D. J., Goddard, J. E., Harmatz, J. S., and Shader, R. I., The neuropsychiatric effects of aspartame in normal volunteers. J Clin Pharmacol. 1990;30(5):454-60.
Larson-Powers, N., and Pangborn, R. M., 1978. Paired comparison and time-intensity measurements of the sensory properties of beverages and gelatins containing sucrose or synthetic sweeteners. J. Food Sci. 43.Leatherhead Food Research. 2011. The global food additives market. 5th ed. Leatherhead (UK): Leatherhead Food Research.
Leon, A. S., Hunninghake, D. B., Bell, C., et al. 1989. Safety of long-term large doses of aspartame. Arch Intern Med. 149:2318-2324.
Lieberman, H. R., Caballero, B., Emde, G. G., and Bernstein, J. G., 1988. The effects of aspartame on human mood, performance, and plasma amino acid levels. In R. J. Wurtman & E. Ritter-Walker (Eds.), Dietary phenylalanine and brain function (pp. 198-200). Boston: Birkhauser.
Lino, C. M., Costa, I. M., Pena, A., Ferreira, R., and Cardoso, S. M., 2008. Estimated intake of the sweeteners, acesulfame-K and aspartame, from soft drinks, soft drinks based on mineral waters and nectars for a group of Portuguese teenage students. Food Addit Contam. 25:1291-6
Lipton, R. B., Newman, L. C., Cohen, J. S., and Solomon, S., 1989. Aspartame as a dietary trigger of headache. Headache. 29:90-2.
Mackey, S. A., and Berlin, C. M. Jr. 1992 Effect of dietary aspartame on plasma concentrations of phenylalanine and tyrosine in normal and homozygous phenylketonuric patients. Clin Pediatr (Phila). 31:394-399.
Maher, T. J., Wurtman, R. J., 1987. Possible neurologic effects of aspartame, a widely used food additive. Environ Health Perspect. 75:689-94.
Mandel, I. D., Grotz, V. L., 2002. Dental considerations in sucralose use. J Clin Dent. 13:116-118.
Mann, S. W., Yuschak, M. M., Amyes, S. J., et al. A combined chronic toxicity/carcinogenicity study of sucralose in Sprague-Dawley rats. Food Chem Toxicol. 38(suppl 2):S71-S89.
Merrill, R. A., and Taylor, M. R., 1985. Saccharin: a case study of government regulation of environmental carcinogens. Virginia Journal of Natural Resources Law. 5: 1-84.
Morgan, R. W., and Wong, O. 1985. A review of epidemiological studies on artificial sweeteners and bladder cancer. Food Chem Toxicol. 23:529-33.
Morrison, A. S., and Buring, J. E., 1980. Artificial sweeteners and cancer of the lower urinary tract. N Engl J Med. 302:537-541.
Mortensen, A., 2006. Sweeteners permitted in the European Union: safety aspects. Scandinavian Journal of Food and Nutrition. 50(3): 104-116.
Mukherjee, A., and Chakrabarti, J., 1997. In vivo cytogenetic studies on mice exposed to acesulfame-K a non-nutritive sweetener. Food and Chemical Toxicology. 35(12), 1177-1179.
Mukherjee, M. and Sarkar, A., 2011. Sugar content in artificial sweetener. Adv Appl Sci Res. 2:407-9.
Mukhopadhyay, M., Mukherjee, A., and Chakrabarti, J., 2000. In vivo cytogenetic studies on blends of aspartame and acesulfame-K. Food Chem Toxicol. 38;75-7.
Nabors, L. O., 2002. Sweet choices: sugar replacements for foods and beverages. Food Technol. 56:28-32.
Newman, L.C., and Lipton, R. B., 2001. Migraine MT-down: an unusual presentation of migraine in patients with aspartame-triggered headaches. Headache. 41:899-901.
Nofre, C. C., and Tinti, J. M., 1996. US Patent 5 480 668.
Nofre, C. C., and Tinti, J. M., 2000. Neotame: discovery, properties, utility. Food Chemistry. 69.
[NTP] Natl. Toxicology Program. 2000. Report on carcinogens. 9th ed. Research Triangle Park, N. C.: NTP.
Olney, J. W., Farber, N. B., Spitznagel, E., et al. 1996. Increasing brain tumor rates: is there a link to aspartame? J Neuropathol Exp Neurol. 55:1115-1123.
Qin, X., 2011. What made Canada become a country with the highest incidence of inflammatory bowel disease: Could sucralose be the culprit? Can J Gastroenterol. 25:511.
Patel, R. M., Sarma, R., and Grimsley, E., 2006. Popular sweetener sucralose as a migraine trigger. Headache. 46:1303-8.
Price, J. M., Biava, C. G., Oser, B. L., Vogin, E. E., Steinfeld, J., and Ley, H. L., 1970. Bladder tumours in rats fed cyclohehylamine or high doses of a mixture of cyclamate and saccharin. Science. 167: 11312.
Renwick, A. G., 1986. The metabolism of intense sweeteners. Xenobiotica. 16:1057-1071.
Roberts, H. J., 1988.. Reactions attributed to aspartame-containing products: 551 cases. J Appl Nutr. 40: 8593.
Roberts, A., Renwick, A. G., Sims, J., et al. 2000. Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol. 38.
Rowan, A. J., Shaywitz, B. A., Tuchman, L., French, J. A., Luciano, D., and Sullivan, C. M., 1995. Aspartame and seizure susceptibility: results of a clinical study in reportedly sensitive individuals. Epilepsia. 36(3):270-5.
SCF, 2000. Opinion of the scientific committee on food on sucralose. European Commission. SCF/CS/ADDS/EDUL/190 Final.
Serra-Majem, L., Bassas, L., Garcia-Glossas, R., Ribas, L., Ingles, C., Casals, I., Saavedra, P., Renwick, A. G., 2003. Cyclamate intake and cyclohexylamine excretion are not related to male fertility in humans. Food Addit Contam 20:1097-104.
Shankar, P., Ahuja, S., and Sriram, K., 2013. Non-nutritive sweeteners: Review and update. Nutrition. 29(11).
Shaywitz, B. A., Sullivan, C. M., Anderson, G. M., et al., 1994(a). Aspartame, behavior, and cognitive function in children with attention deficit disorder. Pediatrics . 93:70-75.
Shaywitz, B. A., Anderson, G. M., Novotny, E. J., et al. 1994(b). Aspartame has no effect on seizures or epileptiform discharges in epileptic children. Ann Neurol. 35:98-103.
Schoenig, G. P., Goldenthal, E. I., Ceil, R. G., Frith, C. H., Richter, W. R., and Carlborg, F. W., 1985. Evaluation of the dose response and in utero exposure to saccharin in the rat. Food Chem. Toxicol. 23: 475-490.
Sims, J., Roberts, A., Daniel, J. W., et al. 2000. The metabolic fate of sucralose in rats. Food Chem Toxicol. 38(suppl 2):S115-S121.
Soffritti, M., Belpoggi, F., Tibaldi, E., Esposti, D. D., and Lauriola, M., 2007. Lifespan exposure to low doses of aspartame beginning during prenatal life increases cancer effects in rats. Environmental Health Perspectives. 115(9), 1293-1297.
Spiers, P. A., Sabounjian, L., Reiner, A., Myers, D. K., Wurtman, J., and Schomer, D. L., 1998. Aspartame: neuropsychologic and neurophysiologic evaluation of acute and chronic effects. Am J Clin Nutr. 68(3):531-7.
Stokes, A. F., Belger, A., Banich, M. T., and Taylor, H., 1991. Effects of acute aspartame and acute alcohol ingestion upon the cognitive performance of pilots. Aviation Space & Environmental Medicine, 62(7), 648-653.
Taylor, J. M., and Friedman. L., 1974. Combined chronic feeding and three-generation reproduction study of sodium saccharin in the rat. Toxicol. Appi. Pharmacol. 29: 154.
Tisdel, M. O., Naces, P. O., Harris, D. L., and Derse, P. H., 1974. Long-term feeding of saccharin in rats. In: G. E. Inglett (ed.). Symposium: Sweeteners, pp. 145-158. Connecticut: Avi Publishing Co..
Trefz, F., de Sonneville, L., Matthis, P., et al., 1994. Neuropsychological and biochemical investigations in heterozygotes for phenylketonuria during ingestion of high dose aspartame (a sweetener containing phenylalanine). Hum Genet. 93:369-374.
[USFDA] U.S. Food and Drug Administration. 2002. Food additives permitted for direct addition to food for human consumption; neotame. Fed Reg 67:45300-10.[USFDA] U.S. Food and Drug Administration. 2014. Food Additives permitted for Direct Addition to Food for Human Consumption; Advantame. Food Reg 21 CFR 172.803. Available at https://www.federalregister.gov/articles/2014/05/21/2014-11584/food-additives-permitted-for-direct-addition-to-food-for-human-consumption-advantame#page-29085
Van Den Eeden, S.K., Koepsell, T.D., Longstreth, W. T. Jr, Van Velle, G., Daling, J.R., McKnight, B. Aspartame ingestion and headaches. AAA 1994; 44:1787-93.
Wetzel, C. R., and Bell, L. N., 1998. Chemical stability of encapsulated aspartame in cakes without added sugar. Food Chemistry. 63(1).
Walker, A. M., Dreyer, N. A., Friedlander, E., et al. 1982. An independent analysis of the National Cancer Institute study on non-nutritive sweeteners and bladder cancer. Am J Public Health. 1982. 72:376-381.
Walton, R. G., 2012. Survey of aspartame studies: correlation of outcome and funding sources. Available at www.dorway.com/peerrev.html.
Wolraich, M. L., Lindgren, S. D., Stumbo, P. J., Stegink, L. D., Appelbaum, M. I., and Kiritsy, M. C., 1994. Effects of diets high in sucrose or aspartame on the behavior and cognitive performance of children. N Engl J Med. 330(5):301-7.
Whelan, A. P., Vega, C., Kerry, J. P., and Goff, H. D., 2008. Physicochemical and sensory optimization of a low glycemic index ice cream formulation. Int J Food Sci Technol. 43:1520–1527.
Whitehouse, C. R., Boullata, J., and McCauley, L. A. 2008. The potential toxicity of artificial sweeteners. AAOHN J. 56:251-9.
Wiseman, J. J., and McDaniel, M. R., 1991. Modification of Fruit Flavours by Aspartame and Sucrose. Journal of Food Science. 56(6).
Wynder, E. L., and Goldsmith, R., 1977. The epidemiology of bladder cancer: a second look. Cancer. 40:1246-1268.
Yilmaz, S., and Ucar, A., 2014. A review of the genotoxic and carcinogenic effects of aspartame: does it safe or note? Cytotechnology. 66(6).
Yu, Y., Hu, J., Wang, P. P., Zou, Y., Qi, Y, Zhao, P., and Xe, R., 1997. Risk factors for bladder cancer: a case-control study in northeast China. Bur J Cancer Prev. 6:363-9.