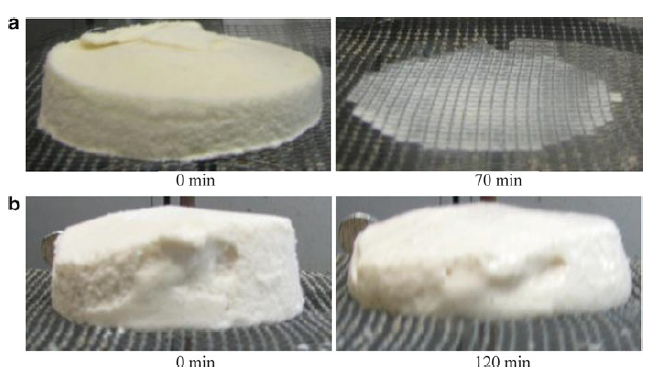
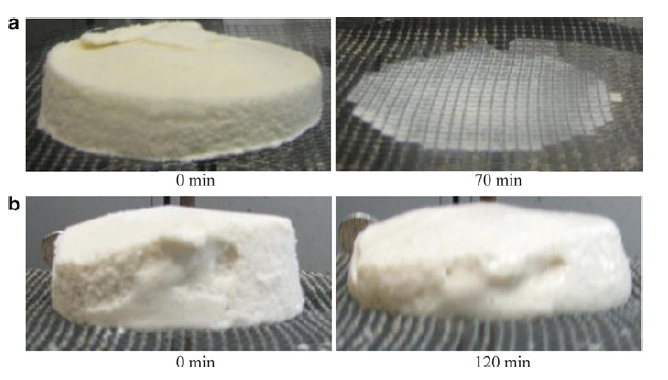
Why does ice cream melt?
A slow melting rate and good shape retention are generally considered desirable qualities in ice cream. Ice cream has three main structural components: air cells, ice crystals, and fat globules, which are dispersed throughout a continuous phase of unfrozen solution (Muse & Hartel, 2003). These components affect the melting rate. Structural attributes include properties of the air phase (overrun and air cell size distribution), fat phase (total fat content, fat globule size distribution and extent of fat destabilisation), ice phase (ice phase volume and ice crystal size distribution), and the continuous phase (viscosity) (Hartel et al., 2003).
YOU MIGHT ALSO LIKE THE FOLLOWING POSTS:
- Cuisinart ICE-100 Compressor Ice Cream and Gelato Maker - Review
- How to make homemade vanilla ice cream - Recipe
- Ice crystal formation and growth in ice cream
- Air bubbles in ice cream
1. AIR PHASE
1.1. OVERRUN
The air that’s whipped in to ice cream (overrun) by the rotating dasher and scraper blades influences the rate of melt down: ice cream containing a high amount of air (high overrun) tends to melt slowly (Goff & Hartel, 2013). Air cells act as an insulator and slow the ability of heat to penetrate into the ice cream and melt the ice crystals, thus reducing the rate of meltdown (Sofjan & Hartel, 2004).
Sakurai et al. (1996) found that ice creams with low overruns melted quickly, whereas ice creams with high overruns began to melt slowly and had a good melting resistance. Similarly, Sofjan & Hartel (2004) found that ice cream made with 80% overrun melted more rapidly than those made with 100% and 120% overrun.
1.2. AIR CELL SIZE DISTRIBUTION
The air cell size distribution also influences melt down, with smaller air cells likely contributing to lower rates of melt down. During dynamic freezing, where the ice cream mix is frozen in an ice cream maker whilst being agitated to incorporate air, air cells start out as large entities but are continually reduced in size by the shear stress, or the imposed force, of the rotating dasher and scraper blades (Goff & Hartel, 2013). Sofjan & Hartel (2004) observed that the ice cream made with 80% overrun had larger air cells and ice crystals than ice creams made with 100% and 120% overrun, which, they noted, may have influenced melt down rates. A similar view is taken by Goff & Hartel (2013) who note that ice cream with larger air cells might be expected to melt more quickly, although they stress that no evidence of this has been published.
There are several factors that influence overrun and air cell size distribution. These include residence time, rotor speed, and viscosity.
1.3. RESIDENCE TIME
The amount of time an ice cream mix spends in the machine during dynamic freezing, the residence time, influences both the overrun and air cell size, with longer residence times resulting in smaller air cells and higher overrun (Chang & Hartel, 2002; Thakur & others, 2005; Kroezen, 1988).
However, residence time also has a significant effect on ice crystal size (Russell et al., 1999; Goff & Hartel, 2013; Drewett & Hartel, 2007; Cook & Hartel, 2010), with longer residence times producing larger ice crystals (Russell et al., 1999). Ice crystal size is a critical factor in the development of smooth texture (Donhowe et al. 1991) with smooth and creamy ice cream requiring the majority of ice crystals to be small, around 10 to 20 µm in size. If many crystals are larger than this, the ice cream will be perceived as being coarse or icy (Drewett & Hartel, 2007; Goff & Hartel, 2013).
Koxholt et al. (2000) note that the dynamic freezing stage must account for competing phenomena as shorter freezing times are needed to produce small ice crystals, but longer freezing times give smaller air cells.
TIP #1To increase overrun and promote the formation of small air cells, leave your ice cream mix churning in your machine for longer. Below are the residence times for domestic machines I’ve tested, along with links to my reviews. You could try increasing these residence times by 5-15 minutes to achieve higher rates of overrun and the dispersion of smaller air cells. Do bare in mind that the longer you leave your ice cream in the machine, the larger the ice crystals are likely to grow and the sandier the texture is likely to be.
Cuisinart ICE 30BC: 1000g batch – 35 minutesLello Musso Pola 5030 Dessert Maker: 1000g batch – 13 minutesLello 4080 Musso Lussino: 693g - 16 minutesCuisinart ICE-100 Ice Cream and Gelato Maker: 800g batch – 32 minutesBreville BCI600XL Smart Scoop Ice Cream Maker: 732g batch – 30 minutesKitchenAid K45SS: 1000g batch – 30 minutesDeLonghi GM6000 Gelato Maker: 600g batch 30 minutes
1.4. ROTOR SPEED
An increase in the rotational speed of the dasher and scraper blades results in a smaller mean bubble diameter (Den Engelsen et al., 2002). In a study on the size of bubbles in foam in a dynamic mixer, Kroezen (1988) found that the mean bubble diameter decreased with increasing rotational speed of the mixer. Similarly, Gido et al. (1989) found that increasing the rotational speed of a dynamic mixer caused a decrease in the average bubble diameter.
Increased rotor speed, however, appears to have a detrimental effect on ice crystal size, although there appears to be conflicting research on the extent of this effect. Hartel (1996) argues that increasing the agitation speed or the number of scraper blades has significant effect on ice crystal formation during the freezing of ice cream. This is because the increased agitation speed causes an increase in the input of heat, which may result in larger ice crystals. Russell et al. (1999) also found that increasing dasher speeds results in an increase in ice crystal size. Drewett & Hartel (2007), however, found only a slight increase, Koxholt et al. (2000) found no effect, and Inoue et al. (2008) found mixed effects on ice crystal size. Cook & Hartel (2010) argue that it's possible that the dasher speed itself is not a direct predictor of ice crystal size. Instead, heat generation by the dasher may give a better correlation with ice crystal size.
Rotor speed is unlikely to be relevant for the home cook because most domestic ice cream machines don’t give you the option of altering the rotor speed. The only domestic ice cream maker I've tested that lets you alter rotor speed is the [amazon text=KitchenAid K45SS Classic Stand Mixer&asin=B00O4XR5QQ&asin[uk]=B000VKHCEQ&asin[ca]=B00O4XR5QQ&asin[de]=B000VKHCEQ&asin[fr]=B0118EAKSS&asin[es]=B000VKHCEQ&asin[it]=B000VKHCEQ].
1.5. VISCOSITY
Hirt et al. (1987) found that an increase in the viscosity of the liquid phase, or a thicker ice cream mix, resulted in a smaller mean air bubble size. Similarly, Den Engelsen et al. (2002) found that a small increase of the viscosity of the liquid ice cream mix yields a decrease of the bubble size. They found, however, that above a certain viscosity, an increase of the large bubbles is observed and the process of bubble formation is apparently retarded.
Stabilisers are known to increase the viscosity of the aqueous phase. Chang & Hartel (2002) found that the addition of stabiliser caused an increase in viscosity and this led to smaller air cells during the early stages of freezing.
2. FAT PHASE
2.1. TOTAL FAT CONTENT
Ice cream containing a high amount of fat tends to melt more slowly. Roland, Philips, and Boor (1999) studied ice cream formulated with different percentages of fat and showed that the melting rate decreases with high fat content. This was verified by Alamprese et al. (2002) who reported that ice cream with higher fat content is softer and shows a slower melting rate. Hyvonn et al. (2003) found similar results and reported that increasing the fat content slightly retarded melting of ice cream in the mouth.
TIP #2To promote a decrease in the melting rate of ice cream, use an ice cream mix with a high fat content. My ice cream base mix contains around 23% fat after it’s been reduced, which I've found to be optimum for the development of smooth and creamy texture. Have a look at my How to make homemade vanilla ice cream - Recipe for the amount of cream needed in homemade ice cream.
2.2. PARTIAL COALESCENCE OF FAT
Fat undergoes partial coalescence, or destabilisation, during dynamic freezing. Partial coalescence occurs when a protruding fat crystal from one fat globule pierces the interfacial film of another globule, forming a largely irreversible connection between the internal phases of the globules (Walstra, 2003). These partially coalesced globules are crucial for the development of smooth texture and resistance to meltdown (Goff, 1997): the rate of meltdown generally decreases as the extent of fat destabilisation increases (Nielsen, 1976; Muse & Hartel, 2004; Goff & Hartel, 2013).
Berger & White (1971) and Berger et al. (1972) proved that fat destabilisation has a significant influence on parameters such as dry appearance, creamy mouthfeel and meltdown behaviour. Similarly, Campbell and Pelan (1998) found that meltdown resistance increased as draw temperature (the temperature at which ice cream is removed from the ice cream machine) decreased due to increased overrun and fat destabilisation, although ice crystals may also have influenced the melt-down rate. Goff & Hartel (2013), however, note that because there are numerous structural factors that influence the rate of meltdown, a direct correlation between partial coalescence and meltdown rate has not always been observed.
The fat globule clusters that form during partial coalescence are mainly responsible for stabilising air bubbles (Walstra, 1989; Chang & Hartel, 2002). This results in the beneficial properties of dryness, smooth texture, and resistance to meltdown (Lin & Leeder, 1974; Buchheiim et al., 1985; Berger, 1990). According to Pelan et al. (1997), it is the stability of air cells that slows down the meltdown rate of ice cream.
Factors affecting both the rate of partial coalescence and air cell stability include protein, emulsifiers, and ageing.
2.2.1. PROTEIN
Proteins, usually present at about 4% in ice cream (Daw & Hartel, 2015), can prevent partial coalescence by adsorbing as thick layers to fat globules (Goff, 1997; Segall & Goff, 1999). These thick protein layers provide significant stabilisation, which prevents the close contact between globules necessary for partial coalescence (Fuller et al., 2015).
Daw & Hartel (2015) showed that the extent of partially coalesced fat decreased and melt rate increased as protein content increased. Similarly, Williams & Dickenson (1995) showed that the stability of an emulsion stabilised by β-lactoglobulin, the major whey protein, increased as the concentration of protein increased, making it less prone to partial coalescence.
TIP #3To promote partial coalescence of fat globules, limit protein content in your mix to around 4% by ensuring that your milk solids-not-fat (the lactose, proteins, minerals, water-soluble vitamins, enzymes, and some minor constituents) content, which is between 34-36% milk protein (Goff & Hartel, 2013), does not exceed around 11% of your mix.
2.2.2. EMULSIFIER
Surfactants (emulsifiers such as monoglycerides, diglycerides, or polysorbate 80) play a critical role in the promotion of partial coalescence (Musselwhite & Walker, 1971; Goff, 1997) by displacing adsorbed proteins from the fat interface.
The addition of polysorbate 80 to ice cream (at levels of 0.02% and above) has been shown to greatly decrease the melting rate of ice cream and to promote shape retention (Cambell et al., 1998; Tharp et al, 1998). Similarly, Muse & Hartel (2004) found that the level of destabilised fat generally increased by increasing levels of polysorbate 80 from 0 and 0.05 to 0.1%.
Flores & Goff (1999) found that added emulsifier resulted in more air incorporation and air was more finely dispersed. Zhang & Goff (2005) found that added emulsifiers increased air bubble stability through promoting partial coalescence of fat, but too much partially coalesced fat led to unstable air bubbles.
TIP #4Add 0.1-0.3% of polysorbate 80 to your mix to promote partial coalescence and increase air bubble stability. Lecithin, which is a naturally occurring emulsifier from egg yolk, acts in similar ways to polysorbate 80 to promote partial coalescence. I’ve found that around 4.3% egg yolks after reduction is optimum in homemade ice cream.
2.2.3. AGEING
Partial coalescence can only occur if fat droplets contain solid particles, nearly always crystals of oil molecules (Goff, 1997; Fredrick et al., 2010). Crystallisation of fat globules takes place during the ageing stage where the ice cream mix is kept at 4°C (39.2°F) for at least 4 hours after it's been pasteurised. About 2/3 of milk fat will crystallise at 4°C (39.2°F) and this has fully crystallised after 4-5 hours of ageing (Adleman & Hartel, 2001). Ice cream mixes which are not aged or formulated without emulsifier do not undergo much partial coalescence and tend to be wet, suffer from less retention of shape and relatively fast meltdown (Goff & Jordan, 1989; Goff & Hartel, 2013).
TIP #5Place your ice cream mix in your fridge and age overnight at 4°C (39.2°F) to promote crystallisation of the fat globules, which is necessary to promote partial coalescence during the dynamic freezing stage.
3. TOTAL SOLIDS
Goff & Hartel (2013) note that low freezing point, or the temperature at which ice cream first begins to freeze, is the primary cause of rapid melting. Li et al. (1997) found hat ice creams with a high amount of total solids (39%) (the sum of the fat, milk solids-not-fat, sugars, stabilisers, and emulsifiers) melted faster than did those ice creams containing low amounts of totals solids (33%). This, they argued, was probably due to the effect of dissolved solids on lowering the freezing point. Similar results were reported by Kurultay et al. (2010) who reported that melting rapidly increased with increasing total solids from 20, to 30, to 40%.
TIP #6I wouldn't recommend lowering the total solids content in homemade ice cream because doing so will likely result in coarse or grainy texture. Commercial ice cream mixes have a relatively low total solids content because the short residence times in commercial machines produce small ice crystals, necessary for smooth texture. A mix with a similar low total solids content produced in a domestic machine would not have the same smooth texture because of the larger ice crystals that would result from the increased residence time. We as home cooks, therefore, have to compensate by increasing the total solids content, primarily the fat content, in our homemade ice cream to develop smooth and creamy texture. My recipes call for around a 55% total solids content after reduction, which I've found optimum for homemade ice cream.
4. ICE CRYSTALS
Muse & Hartel (2004) note that larger ice crystals increase meltdown rate, possibly because larger ice crystals take longer to melt than smaller ones.
5. VISCOSITY
Viscosity of the serum phase is another factor that significantly slows meltdown rate (Muse & Hartel, 2004). A more viscous serum phase, or a thicker ice cream mix, will drain more slowly through the lamellar spaces between air cells, thus reducing meltdown. El-Nagar et al. (2002) found that an increase in serum viscosity resulted in a decrease in meltdown rate. Similarly, Huppertz et al. (2011) found that the increased viscosity that resulted from protein denaturation reduced meltdown of the finished ice cream.
6. SWEETENERS
The type and amount of sweetener also affects the melting rate of ice cream (Muse & Hartel, 2004). Sweeteners lower the freezing point of an ice cream mix, which leads to an increased rate of melting (Goff & Hartel, 2013).
Junior & Lannes (2011) found that formulations added with fructose syrup generally presented a higher melting rate when compared to ice creams made with glucose syrup, a probable cause being the impact of fructose syrup on the freezing point of the mixes. Muse & Hartel (2004) tested the effects of three types of sweeteners, sucrose, 42 dextrose equivalent high fructose corn syrup, and 20 dextrose equivalent corn syrup, and three levels of the emulsifier (0, 0.05, and 0.1%) polysorbate 80 on melting rate and hardness. They found that ice cream made with 20 dextrose equivalent corn syrup had the slowest melting rate, whereas ice ream made with 42 dextrose equivalent high fructose corn syrup had the fastest melting rates. They also reported that ice creams made with 20 DE corn syrup generally had the highest levels of destabilised fat, which was attributed to the high viscosity and percentage of ice during freezing, which lead to high shear forces and enhanced fat destabilisation.
I hope this cumbersome post helps. I'd be happy to answer any questions so do get in touch and say hi! All the best, Ruben :)
References:
Alderman R., and Hartel, R. W., 2001. Lipid crystallization and its effect on the physical structure of ice cream. In: Garti, N., Sato, K., (eds) Crystallization processes in fats and lipid systems. New York: MarcelDekker. pp 381–427.
Alamprese, C., Foschino, R., Rossi, M., Pompei, C., and Savani, L., 2002. Survival of lactobacillus johnsonii la1 and influence of its addition in retail-manufactured ice cream produced with different sugar and fat concentrations. International Dairy Journal. 2, 201-208.
Berger, K. G., 1990. Ice cream. In Larson, K., and Friberg, S., Food Emulsions, 2nd ed. New York: Marcel Dekker Inc.
Berger, K. G., and White, G., W., 1971. An electron microscopical investigation of fat destabilization in ice cream. Journal of Food Technology. 6, 285–294.
Berger, K. G., Bullimore, B. K., White, G. W., and Wright, W. B., 1972. The structure of ice cream. Dairy Ind. 37, 419–425, 493–497.
Buchheim, W., Barfod, N. M., and Krog, N., 1985. Relation between microstructure, destabilization phenomena and rheological properties of shippable emulsions. Food Microstructure. 4, 221-232.
Campbell, I. J., and Pelan, B. M. C., 1998. The influence of emulsion stability on the properties of ice cream. Pages 25–36 in Ice Cream: Proceedings of the International Symposium held in Athens, Greece, 18–19 September 1997. W. Buchheim, ed. International Dairy Federation, Brussels, Belgium.
Cook, K. L. K., & Hartel, R. W., 2010. Mechanisms of Ice Crystallisation in Ice Cream Production. Comprehensive Reviews in Food Science and Food Safety. 9(2).
Chang, Y., and Hartel, R. W., 2002. Development of air cells in a batch ice cream freezer. Journal of Food Engineering. 55, 71-78.
Daw, E., and Hartel, R. W., 2015. Fat destabilization and melt-down of ice creams with increased protein content. International Dairy Journal. 43, 33-41.
Den Engelsen, C. W., Isarin, J. C., Gooijer, H., Warmoeskerken, M. M. C. G., and Groot Wassink, J., 2002. Bubble size distribution of foam. AUTEX Research Journal. 2(1).
Donhowe, D. P., Hartel R. W., and Bradley R. L., 1991. Determination of ice crystal size distributions in frozen desserts. Journal of Dairy Science. 74.
Drewett, E. M., & Hartel, R. W., 2007. Ice crystallisation in a scraped surface freezer. Journal of Food Engineering 78(3).
El-Nagar, G., Clowes, G., Tudorica, C. M., Kuri, V., and Brennan, C. S., 2002. Rheological quality and stability of yog-ice cream with added inulin. International Journal of Dairy Technology. 55(2), 89–93.
Flores, A. A., and Goff, H. D., 1999. Ice crystal size distributions in dynamically frozen model solutions and ice cream as affected by stabilizers. Journal of Dairy Science. 82, 1399–1407.
Fredrick, E., Walstra, P., and Dewettinck, K., 2010. Factors governing partial coalescence in oil-in-water emulsions. Advances in Colloid and Interface Science. 153(1)
Fuller, G. T., Considine, T., Golding, M., Matia-Merino, L., and MacGibbon, A., 2015. Aggregation behaviour of partially crystalline oil-in-water emulsions: Part II – Effect of solid fat content and interfacial film composition on quiescent and shear stability. Food Hydrocolloids. 51.
Gido S. P., Hirt, D. E., Montgomery S. M., Prud’home, R. K., and Renbenfeld, L., 1989. Foam bubble size measured using image analysis before and after passage through a porous medium. J. Dispersion Science and Technology. 10, 785-793.
Goff, H. D., 1997. Instability and Partial Coalescence in shippable Dairy Emulsions. Journal of Dairy Science. 80, 2620-2630.
Goff, H. D., and Jordan, W. K., 1989. Action of emulsifiers in promoting fat destabilization during the manufacture of ice cream. Journal of Dairy Science. 72(18).
Goff, H. D., and Hartel R. W., 2013. Ice Cream. Seventh Edition. New York: Springer.
Hartel, R. W., 1996. Ice crystallisation during the manufacture of ice cream. Trends in Food Science & Technology. 7(10).
Hartel, R. W., Muse, M., and Sofjan, R., 2003. Effects of structural attributes on hardness and melting rate of ice cream. In Proceedings of the 2nd IDF Ice Cream Symposium, Thessoliniki, Greece. (in press)
Hirt, D. E., Prud’homme, R. K., and Rebenfeld, L., 1987. Characterization of foam cell size and foam quality using factorial design analyses. Journal of Dispersion Science and Technology. 8.
Huppertz, T., Smiddy, M. A., Goff, H. D., Kelly, A. L., 2011. Effect of high pressure treatment of mix on ice cream manufacture. International Dairy Journal. 21, 718–726.
Hyvonen, L., Linna, M., Tutorial, H., and Dijksterhuis, G., 2003. Perception of Melting and Flavor Release of Ice Cream Containing Different Types and Contents of Fat. Journal of Dairy Science. 86, 1130-1138.
Inoue, K., Ochi, H., Taketsuka, M., Saito H., Sakurai, K., Ichihashi, N., Iwatsuki, K., Kokubo, S., 2008. Modelling of the effect of freezer conditions on the principal constituent parameters of ice cream by using response surface methodology. Journal of Dairy Science. 91(5), 1722-32.
Junior, E. D. S., and Lannes, S. C. D. S., 2011. Effect of different sweetener blends and fat types on ice cream properties. Cienc. Tecnol. Aliment., Campinas. 31(1), 217-220.
Koxholt, M., Eisenmann, B., Hinrichs, J., 2000. Effect of process parameters on the structure of ice cream. Eur Dairy Mag. 1, 27-30.
Kroezen, A. B. J., 1988. Flow properties of foam in rotor-stator mixers and distribution equipment. Ph.D Thesis, University of Twente, Enschede, The Netherlands.
Kurultay, S., Oksuz, O., and Gokcebag, O., 2010. The influence of different total solid, stabiliser, and overrun levels in industrial ice cream production using coconut oil. Journal of Food Processing and Preservation. 34, 346-354.
Li, A., Marshall R. T., Heymann H., and Fernando, L. 1997. Effect of milk fat content on fl avor perception of vanilla ice cream. Journal of Dairy Science. 80, 3133–3141.
Lin, P. M., and Leeder, J. G., 1974. Mechanisms of emulsifier action in an ice cream system. Journal of Food Science. 39, 108-111.
Muse, M. R., and Hartel, R. W., 2004. Ice Cream Structural Elements that Affect Melting Rate and Hardness. Journal of Dairy Science. 87, 1-10.
Musselwhite, P. R., and D. A. Walker. 1971. The effect of the colloidal state of the emulsion on ice cream structure. Journal of Texture Studies. 2, 110.
Nielsen, B. J., 1976. Function and evaluation of emulsifiers in ice cream and whippable emulsions. Gordian 76, 220.
Pelan, B. M. C., Watts, K. M., Campbell, I. J., and Lips, A. 1997. The stability of aerated milk protein emulsions in the presence of small molecule surfactants. Journal of Dairy Science. 80. 2631–2638
Roland, A. M., Philips, L. G., and Boor, K. J., 1999. Effects of fat content on the sensory properties, melting, color, and hardness of ice cream. Journal of Dairy Science. 82, 32-38
Russell, A. B., Cheney, P. E., & Wantling, S. D., 1999. Influence of freezing conditions on ice crystallisation in ice cream. Journal of Food Engineering. 29.
Sakurai, K., Kokubo, S., Hakamata, K., Tomita, M., and Yoshida, s., 1996. Effect of production conditions on ice cream melting resistance and hardness. Milchwissenschaft. 51(8), 451–454.
Segall, K. I., and Goff, H. D., 1999. Influence of adsorbed milk protein type and surface concentration on the quiescent and shear stability of butteroil emulsions. International Dairy Journal. 9, 683 -691.
Sofjan, R. P., and Hartel, R. W., 2003. Effects of overrun on structural and physical characteristics of ice cream. International Dairy Journal. 14, 255-262
Thakur, R. K., Vial, C., Djelveh G., 2005. Combined effects of process parameters and composition on foaming of dairy emulsions at low temperature in an agitated column. Journal of Food Engineering. 68(3), 335–47.
Tharp. B. W., Forrest, B., Swan, C., Dunning, L., and Hilmoe, M., 1998. Basic factors affecting ice cream meltdown. Pages 54–64 in Ice Cream: Proceedings of the International Symposium held in Athens, Greece, 18–19 September 1997. W. Buchheim, ed. International Dairy Federation, Brussels, Belgium.
Walstra, P., 1989. Principles of foam formation and stability. In Wilson, A. J., (Ed), Foams: Physics, Chemistry and Structure. Berlin: Springer.
Walstra, P., 2003. Physical chemistry of foods. New York: Marcel Dekker.
Williams, A., and Dickinson, E., 1995. Shear-induced instability of oil-in-water emulsions. Page 252 in Food Macromolecules and Colloids. E. Dickinson and D. Lorient, ed. R. Soc. Chem., London, England.
Zhang, Z., and Goff, H. D., 2005. On fat destabilization and composition of the air interface in ice cream containing saturated and unsaturated monoglyceride. International Dairy Journal. 15, 495-500.