Locust Bean Gum in Ice Cream
12 MINUTE READStabilizers used in ice cream production fall into the following categories: seed gums; microbial gums; seaweed extracts; proteins; plant exudates; pectins; and cellulosics. In this post, we'll be looking at locust bean gum, a seed gum. Along with guar gum, carboxymethyl cellulose (also known cellulose gum), and carrageenan, it is the most frequently used stabilizer for regular ice cream formulations (Goff & Hartel, 2013).
You can click here to read my comprehensive review of why stabilizers are added to ice cream.
[toc]
1. What is Locust Bean Gum?
Locust bean gum (Ceratonia silliqua), also known as carob bean gum, is a white to yellowish-white flour obtained by crushing the endosperm of the the seeds of the carob tree Ceratonia siliqua L. It is comprised mainly of a galactomannan polysaccharide composed of the sugars mannose and galactose (Pollard et al., 2008; Dakia et al., 2008). Galactomannans are naturally-occurring polysaccharides (carbohydrates whose molecules consist of a number of sugar molecules bonded together) known for their water-holding, thickening, gelling, binding, suspending, emulsifying, and film-formation properties when hydrated in water.
2. Processing
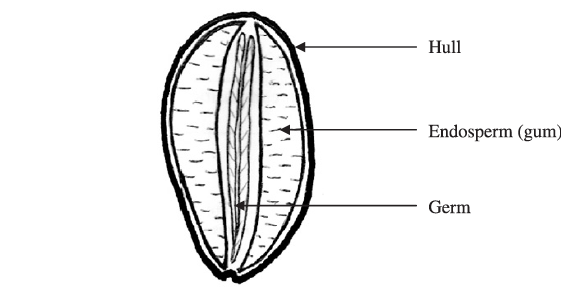
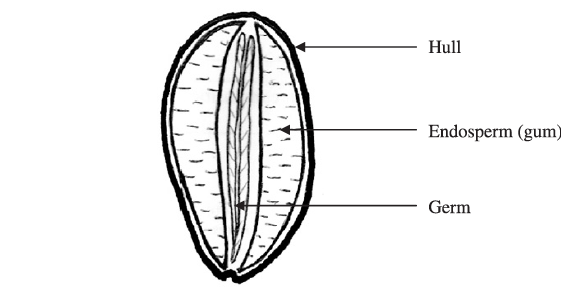
Carob trees yield large brown fruits known as carob pods, which contain 10-15 oval shaped carob seeds or kernels. These seeds are composed of three major parts, the hull (or outer husk) (30-33%), the germ (23-25%), and the endosperm (42-46%), with approximately 80% by weight of the endosperm being composed of galactomannan and the rest corresponding to proteins and impurities (Barak & Mudgil, 2014).
Processing first involves the removal of the outer husk from the seeds, which is achieved either by water-dehulling, acid-peeling, or high-temperature roasting. In the water-dehulling treatment, whole carob seeds are immersed in boiling water for about an hour. The seeds are then removed from the water, washed, and the husk easily broken and manually separated from the endosperm. This process produces a gum that is yellowish in colour. In the acid-peeling process, carob seeds are treated with sulphuric acid at elevated temperatures to carbonise the seed coat. The remaining fragments of the seed coat are then removed from the endosperm by washing and brushing. This process produces a gum that is white in colour. Comparing the water dehulling and acid-peeling treatments, Dakia et al. (2008) found that the acid-peeling process produced locust bean gum with the best thickening properties, according to its higher values of galactomannan content, solubility at high temperature, molecular size, and intrinsic viscosity. Carob kernels can also be roasted in a rotating furnace where the husk more or less pops off. This process produces locust bean gum that is darker in colour due to the elevated temperatures. However, as we'll see in section 3.1 below, elevated temperatures during processing reduce the potential of locust bean gum to develop high viscosities, and thus lower its inherent value.
Following the removal of the husk, the seeds are broken and the germ is separated from the endosperm. The separated endosperm is then milled to the desirable granulation, sieved, graded, packaged, and marketed as locust bean or carob gum.
2.1 Optimum treatment for the production of locust bean gum
Pollard et al. (2008) found the following treatment to be optimum for producing locust bean gum flour with the highest possible viscosity and the least amount of galactomannan degradation: pre-soak seeds at 90°C (194°F) for 8 min; swell for 3 days; cut the swollen seeds with a scalpel and isolate the endosperms by hand; store the endosperms in water for an additional 30 min to increase hydration to about 70%; mill while hydrated, whole or pre-cut, using the centrifugal mill. The researchers found that gum flours produced by this optimised method had the highest average molecular weight, intrinsic viscosity, and solution viscosity.
3. Locust bean gum quality
Seed-processing can provide a gum powder of lower or higher quality depending on the temperature the endosperm is exposed to, the size of the milled endosperm particles, and the impurities and galactomannan contents (Glicksman, 1969; Fox, 1992; Pollard et al., 2008).
3.1. Endosperm temperature
Exposing the endosperm to high temperatures during processing is recognised to reduce the potential of locust bean gum to develop high viscosities, and thus lower its inherent value (Owen et al., 1992; Pollard et al., 2008). Pollard et al. (2008) found that exposing the seed to high temperatures to loosen the husk and aid in separating the main seed components caused a significant loss of solubility, particularly for temperatures above 65°C (149°F). High temperatures generated by dry milling of hard endosperms were also found to result in the degradation of the galactomannan. The researchers found that varying the temperature and hydration state of the endosperms was an effective way to control the temperature during milling. Higher solution viscosities were obtained when the endosperm was cooled on ice or liquid nitrogen before milling, as opposed to those milled while hot. The greatest improvement was found by hydrating the endosperms before milling in their ‘rubbery’ state.
3.2 Size of the milled endosperm particles
Kok (2007) showed that, in general, higher quality refined locust bean gum is lighter in colour and smaller in particle size (like flour), in comparison to lower quality crude locust bean gum.
3.3. Impurities and galactomannan content
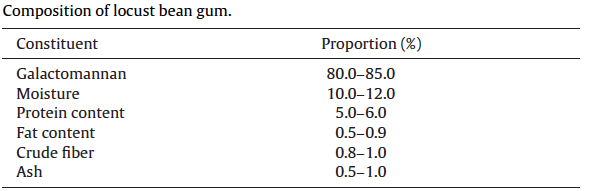
Because of the extreme hardness of the seeds, the difficulty in removing the husk, and the difficulty in separating the husk, germ, and endosperm during processing without cross-contamination, the milled crude gum is not perfectly pure but contains small amounts of the hull and germ, which are deemed impurities. The degree of separation of the husk, germ, and endosperm, therefore determines the quality of the gum, with a higher degree of separation producing gums with fewer impurities and a higher galactomannan content. Gums containing a higher amount of soluble galactomannan and a lower amount of impurities produce samples with a much higher viscosity (Kok et al., 1999; Dakia et al., 2008). The typical composition of a high purity locust bean gum is moisture 10-13%, protein 5%, ash 1%, fibre 1%, and the remainder is galactomannan 80-85% (Maier et al., 1993). A lower-grade locust bean gum can have a galactomannan content of only 72% (Kok et al., 1999).
3.3.1. Clarifying crude locust bean gum
Milled locust bean gum powder can be clarified (extracted, purified) to increase the proportion of galactomannans and to eliminate odours, impurities, and endogenous enzymes (da Silva & Goncalves, 1990). However, locust bean gum is often not commercially available in purified forms and contains significant amounts of non-galactomannan impurities (Kok, 2007).
The clarifying process starts by dispersing the crude locust bean gum in hot water, soda, or acetic acid. This solution is then either filtered or undergoes a centrifugation step to remove insoluble substances. Azero & Andrade (2002) recommend centrifugation as the purification method due to it producing locust bean gum with a substantially higher intrinsic viscosity than locust bean gum purified by filtration. Following either filtration or centrifugation, galactomannans are recovered by precipitation using solvents such as isopropanol, ethanol, or methanol, followed by filtering, drying, and milling to obtain fine particle size powder of purified carob bean gum. Residual amounts of ethanol or isopropanol, however, also act as impurities in locust bean gum powder (Barak & Mudgil, 2014).
4. Use in ice cream
4.1 To increase mix viscosity
The most significant property of seed gums is their ability to hydrate and form very viscous solutions. Viscosity can be loosely defined as the thickness of a liquid, with thicker liquids having higher viscosities (honey has a higher viscosity than water for example). In general, as the viscosity of an ice cream mix increases, the smoothness of texture and resistance to melting increases (Marshall et al., 2003). Locust bean gum is able to form a very viscous solution at relatively low concentrations, although is generally less viscous than guar gum (Barak & Mudgil, 2014).
4.2 To prevent ice and lactose crystal growth during storage
During storage, ice and lactose crystals grow and undergo recrystallisation. Recrystallisation is defined as “any change in number, size, shape… of crystals [during storage]” (Fennema, 1973) and basically involves small crystals disappearing, large crystals growing, and crystals fusing together. Ice and lactose crystal growth and recrystallisation during storage eventually leads to coarse and icy texture. Stabilizers are added to ice cream mainly to extend shelf life by retarding or reducing ice and lactose crystal growth and recrystallisation during storage (or masking the effects of crystal growth), with the most widely used stabilizers for this purpose being guar and locust bean gum (Adapa et al., 2000; Marshall et al., 2003; Bahram-Parvar & Tehrani, 2011). Locust bean gum has been shown to reduce recrystallisation rates better than guar gum (Wittinger & Smith, 1986; Goff et al., 1999; Sutton & Wilcox, 1998). Sutton & Wilcox (1998) showed that the inhibition of recrystallisation by locust bean and guar gum was concentration dependent up to a level of about 0.3%, after which further addition did not result in further inhibition.
4.2.1. Heat shock
Locust bean gum is unsurpassed for its ability to retard or reduce ice and lactose crystal growth and recrystallisation during periods of temperature fluctuation, known as heat shock, where ice crystal growth and recrystallisation occur significantly faster (Donhowe & Hartel, 1996; Kok, 2007). Heat shock occurs when ice cream is left at room temperature for extended periods of time and then re-frozen, or when an ice cream freezer is constantly opened and closed.
4.3. How much locust bean gum is used in ice cream?
Locust bean gum is used at 0.1-0.2% levels in ice cream (Bahram-Parvar & Tehrani, 2011) and can be used alone or in combination with guar gum. Goff and Hartel (2013) note that individual stabilizers seldom perform all of the desired functions; each has a particular effect on body, texture, meltdown, and stability in storage. Therefore, to gain synergism in function, individual substances are usually combined as mixtures of stabilizers and emulsifiers. Usually, 0.2-0.5% of a stabilizer/emulsifier blend is used in an ice cream mix.
4.4 Heating and Hydration
Locust bean gum is only slightly soluble in cold water (i.e. it doesn't dissolve very well) and must therefore be heated to about 80°C (176°F) for 20 - 30 minutes for complete solubilisation and full viscosity in water (Garcia-Ochoa & Casas, 1992). Heating above 80°C (176°F) may cause a reduction in the viscosity of the solution (Barak & Mudgil, 2014). Hydration of about 2 hours is then required in order to reach maximum viscosity (Srivastava & Kapoor, 2005).
4.5. Use with Carrageenan
Locust bean gum, along with guar gum, carboxymethyl cellulose, and xanthan, is incompatible with milk proteins in the ice cream mix and thus will cause an undesirable phase separation known as ‘wheying off’. Wheying off refers to the leaking of a clear watery serum layer during the melting of ice cream, which has an undesirable appearance (Goff & Hartel, 2013). Xanthan gum is the most incompatible with milk proteins, followed by guar gum, and locust bean gum (Thaiudom & Goff, 2003). Carrageenan is frequently used with locust bean gum to retard or prevent this phase separation (Bourriot et al., 1999; Langendorff et al., 2000; Thaiudom & Goff, 2003). It is included in most blended stabilizer formulations at usage rates of 0.01-0.015% (Goff & Hartel, 2013). At higher concentrations (0.05%), carrageenan begins to gel and fail to function well (Thaiudom & Goff, 2003).
5. Summary
Locust bean gum is a white to yellowish-white flour obtained by crushing the endosperm of the the seeds of the carob tree. It is used as a stabiliser in ice cream primarily to increase mix viscosity and to inhibit ice and lactose crystal growth during storage, especially during periods of temperature fluctuation. It is used in ice cream formulations at levels of 0.1-0.2%, needs to be heated to about 80°C (176°F) for 20-30 minutes for complete solubilisation and full viscosity, and must then be left in the mix for about 2 hours to reach maximum viscosity. In general, higher quality locust bean gum is lighter in colour and smaller in particle size. Locust bean gum containing a higher amount of soluble galactomannan (between 80-85%) and a lower amount of impurities produces a much more viscous solution. Carrageenan is frequently used with locust bean gum at usage rates of 0.01-0.015% to retard or prevent the undesirable phase separation caused by the incompatibility of locust bean gum with milk proteins.
6. References
Adapa, S., Schmidt, K. A., Jeon, I. J., Herald, T. J., and Flores, R. A., 2000. Mechanisms of ice crystallization and recrystallization in ice cream: a review. Food Reviews International, 16(3), 259–271.
Azero, E. G., and Andrade, C. T., 2002. Testing procedures for galactomannan purification. Polymer Testing, 2(5), 551–556.
Bahram-Parvar, M., Haddad Khodaparast, M. H., and Razavi, S. M. A., 2009. The effect of Lallemantia royleana (Balangu) seed, palmate-tuber salep and carboxymethylcellulose gums on the physiochemical and sensory properties of typical soft ice cream. International Journal of Dairy Technology, 62, 571–576.Bahram-Parvar, M., and Tehrani, M. M., 2011. Application and Functions of Stabilizers in Ice Cream. Food Reviews International, 27:4, 389-407.
Barak, S., and Mudgil, D., 2014. Locust bean gum: Processing, properties and food applications—A review. International Journal of Biological Macromolecules, 66. 74–80.
Bourriot, S., Garnier, C., and Doublier, J. L., 1999. Micellar-caseine k-carrageenan mixtures. I. Phase separation and ultrastructure. Carbohydrate Polymers, 40, 145e157.
da Silva, J. A. L., and Goncalves, M. P., 1990. Studies on a purification method for locust bean gum by precipitation with isopropanol. Food Hydrocolloids, 4, 277–287.
Dakiaa, P. A., Bleckerb, C., Roberta, C., Watheleta, B., and Paquota, M., 2008. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hydrocolloids, 22 807-818.
Donhowe, D. P., Hartel R. W., and Bradley R.L., 1991. Determination of ice crystal size distributions in frozen desserts. Journal of Dairy Science. 74.
Fox, J. E. Seed gums. In: A. Imeson, ed. 1992. Thickening and gelling agents for food (pp. 153–169). London: Chapman and Hall.
Garcia-Ochoa, F., and Casas, J. A., 1992. Viscosity of locust bean (Ceratonia siliqua) gum solutions. Journal of the Science of Food and Agriculture, 59, 97–100.
Glicksman, M., 1969. Gum technology in the Food Industry. Academic Press; New York.
Goff H. D., Ferdinando, D., and Schorsch, C., 1999. Fluorescence microscopy to study galactomannan structure in frozen sucrose and milk protein solutions. Food Hydrocoll, 13:353–364.
Goff, H. D., and Hartel R. W., 2013. Ice Cream. Seventh Edition. New York Springer.
Kok, S., Hill, S. E., and Mitchell, J. R., 1999. A comparison of the rheological behaviour of crude and refined locust bean gum preparations during thermal processing. Carbohydrate Polymers, 38. 261–265.
Kok, S., 2007. A comparative study on the compositions of crude and refined locust bean gum: In relation to rheological properties. Carbohydrate Polymers. 70, 68-76.
Langendorff, V., Cuvelier, G., Michon, C., Launay, B., Parker, A., and De Kruif, C. G., 2000. Effects of carrageenan type on the behaviour of carrageenan/milk mixtures. Food Hydrocolloids, 14, 273-280.
Maier, H., Anderson, M., Karl, C., and Magnuson, K., 1993. Industrial Gums – Polysaccharidesand Their Derivatives, Academic Press, New York, pp. 181–226.
Marshall, R. T., Goff, H. D., and Hartel R. W., 2003. Ice cream (6th ed). New York: Kluwer Academic/Plenum Publishers.
Owen, S. R., Tung, M. A., and Paulson, A. T., 1992. Thermal studies of food polymer dispersions. Journal of Food Engineering, 16, 39–53.
Pollard, M. A., Kelly, R., Fischer, P. A., Windhab, E. J., Eder, B., and Amado, R., 2008. Investigation of molecular weight distribution of LBG galactomannan for flours prepared from individual seeds, mixtures, and commercial samples. Foof Hydrocolloids, 22. 1596-1606.
Sutton, R., and Wilcox, J., 1998. Recrystallization in ice cream as affected by stabilizers. Journal of Food Science, 63, 104–110.
Srivastava, M., and Kapoor, V. P., 2005. Seed Galactomannans: An Overview. Chemistry & Biodiversity, 2(3). 295-317.
Thaiudom, S., and Goff, H. D., 2003. Effect of k-carrageenan on milk protein polysaccharide mixtures. International Dairy Journal, 13, 763-771.
Wittinger, S. A., and Smith, D. E., 1986. Effect of sweeteners and stabilizers on selected sensory attributes and shelf life of ice cream. J Food Sci, 51(6):1463–1466, 1470.