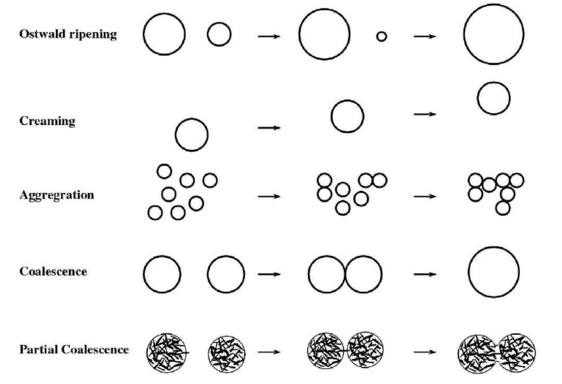
Partial coalescence of the ice cream fat emulsion
Ice cream is an oil-in-water emulsion in which an oil phase, usually consisting of milk fat, is dispersed into a continuous aqueous phase (Fredrick et al., 2010). Emulsions are thermodynamically unstable, meaning that the oil and water will separate due to high interfacial tension between oil and water surfaces.
Immediately following homogenisation, where the fat phase is broken down into numerous small droplets, fat droplets are devoid of any protective membrane. Proteins quickly adsorb to the surface of these small fat droplets, forming a membrane around them. This membrane reduces the interfacial tension between the oil and water surfaces, stabilising the fat droplets and preventing them from coming together (Dickenson, 2003; Goff, 1997).
1. STABILITY OF ICE CREAM EMULSIONS
An ice cream emulsion should be stable prior to freezing but unstable enough so that partial coalescence occurs during the dynamic freezing stage, where the ice cream mix is frozen in a machine whilst being agitated to incorporate air (Goff, 1997). Partial coalescence occurs when a protruding fat crystal from one fat globule pierces the interfacial film of another globule, forming a largely irreversible connection between the internal phases of the globules (Walstra, 2003). These partially coalesced globules are crucial for the development of smooth texture and resistance to meltdown (Goff, 1997).
Goff & Hartel (2013) note that ice cream will suffer from poor shape retention and rapid meltdown if sufficient partial coalescence does not occur, whereas too much partial coalescence may lead to the formation of visible fat granules in the ice cream and ice cream that does not melt within a reasonable time at ambient temperatures.
2. PROMOTING PARTIAL COALESCENCE
To achieve the optimal level of partial coalescence, mix ingredients (protein, emulsifier, and fat) and processing steps (ageing, shear forces during freezing, and homogenisation) need to be controlled.
2.1. PROTEIN
Proteins, usually present at about 4% in ice cream (Daw & Hartel, 2015), can prevent partial coalescence by adsorbing as thick layers to fat globules (Goff, 1997; Segall & Goff, 1999). These thick protein layers provide significant stabilisation, which prevents the close contact between globules necessary for partial coalescence (Fuller et al., 2015).
Daw & Hartel (2015) showed that the extent of partially coalesced fat decreased and melt rate increased as protein content increased. They noted that in particular, whey proteins from added whey protein concentrate were most effective at reducing partial coalescence. Similarly, Williams & Dickenson (1995) showed that the stability of an emulsion stabilised by β-lactoglobulin, the major whey protein, increased as the concentration of protein increased, making it less prone to partial coalescence. This was because of the amount of protein adsorbed at the fat interface.
Generally, whey protein stabilised fat droplets are less prone to fat destabilisation (Segall & Goff, 1999).
TIP#1To promote partial coalescence of fat globules during the dynamic freezing stage, limit protein content in your mix to around 4%. Click here to have a look at the quantity of protein I use in my vanilla bean ice cream recipe.
2.2. EMULSIFIERS
Surfactants (emulsifiers such as monoglycerides, diglycerides, or polysorbate 80) play a critical role in the promotion of partial coalescence in emulsions (Musselwhite & Walker, 1971; Goff, 1997) by displacing adsorbed proteins from the fat interface. Muse & Hartel (2004) found that the level of destabilised fat generally increased by increasing levels of polysorbate 80 from 0 and 0.05 to 0.1%.
In emulsions where both surfactants and proteins are present, the former become preferentially adsorbed to the surface of the fat droplets immediately after homogenisation (Dickinson & Gelin, 1992; Euston et al., 1995.), displacing most, but not all, of the protein present. These added surfactants lower the interfacial tension between the fat and the water phases more than do proteins, making the fat droplets more susceptible to partial coalescence during freezing (Goff & Jordan, 1989; Goff et al., 1987; Goff, 1997). About 0.1-0.3% emulsifiers are typically added to an ice cream mix to help destabilise the emulsion (Daw & Hartel, 2015).
Lecithin, which is a naturally occurring emulsifier from egg yolk, also affects the fat-water interface by coexisting with milk proteins at interfaces (Daw & Hartel, 2015). Dickinson (2003) noted that in combination with whey protein, lecithin does not predominate at the interface but merely reduces the strength of the protein-protein interactions, allowing for increased surface mobility and thinning of the whey protein in the absorbed layer.
TIP#2Add 0.1-0.3% emulsifier to your mix to help destabilise the emulsion and promote partial coalescence.
2.3. TOTAL FAT
Hinrichs & Kessler (1997) showed that emulsions with a high fat volume fraction are more susceptible to partial coalescence than those with a low fat content.2.4 SWEETENERThe type of sweetener used can influence the rate of partial coalescence. Muse & Hartel (2004) tested the effects of three types of sweeteners, sucrose (SUC), 42 dextrose equivalent high fructose corn syrup (HFCS), and 20 dextrose equivalent corn syrup (CS), as well as three levels of the emulsifier polysorbate 80, on melting rate and hardness. They found that ice cream made with the CS generally had the highest levels of destabilised fat. This they attributed to the high viscosity, or the resistance of a liquid to flow, and percentage of ice during freezing, which led to high shear forces and enhanced fat destabilisation.
2.5. AGEING
Partial coalescence can only occur if fat droplets contain solid particles, nearly always crystals of oil molecules (Goff, 1997; Fredrick et al., 2010). Crystallisation of fat globules takes place during the ageing stage where the ice cream mix is kept at 4°C for at least 4 hours after it has been pasteurised and homogenised. About 2/3 of milk fat will crystallise at 4°C and this has fully crystallised after 4-5 hours of ageing (Adleman & Hartel, 2001). The resulting solid fat content, the morphology and size of the crystals and their internal arrangement during freezing will greatly affect the partial coalescence stability of the fat globules (Darling, 1982).
Ice cream mixes which are not aged or formulated without emulsifier do not undergo much partial coalescence and tend to be wet and lacking in body when frozen (Goff & Jordan, 1989).
TIP #3Place your ice cream mix in your fridge and age overnight at 4°C to promote crystallisation of the fat globules, which is necessary for partial coalescence of the fat globules during the dynamic freezing stage.2.6. SHEAR FORCES DURING THE DYNAMIC FREEZING STAGE During the dynamic freezing stage, the shear forces from the rotating dasher bring partially crystalline fat globules together, resulting in aggregated clusters of fat globules (Goff & Hartel, 2013). Shear forces affect the occurrence and the rate of partial coalescence, with higher shearing action of the scraper blades resulting in greater partial coalescence (Fredrick et al., 2010; Goff & Hartel, 2013).
2.7. HOMOGENISATION
Several studies have investigated whether emulsions with improved functional behaviour can be created by controlling the surfactants present during homogenisation. These have included selective homogenisation, where an ice cream mix is split into two separate batches with varying amounts of protein, homogenised separately, and then combined prior to freezing.
Musselwhite & Walker (1971) divided an ice cream mix (10.2% butterfat, 12% milk solids non-fat, 14% sugar, 0.6% gelatine, and 63.2% water) into two separate batches: batch 1 contained only non-fat milk solids and water and batch 2 non-fat milk solids, sugar, gelatine, fat, and water. Protein amounts varied between batches with batch 1 containing 87.5% of the non-fat milk solids and batch 2 only 12.5%. Both batches were homogenised separately and then combined prior to freezing. Musselwhite & Walker found that by homogenising the fat in the presence of only a portion of the milk solids, they were able to influence adsorption at the fat interface by limiting the availability of adsorbing material.
Similarly, Goff et al. (1989) examined fat destabilisation resulting from a selective homogenisation of the ingredients desired at the interface. They prepared one mix with 75% whey protein concentrate (WPC), a second where the WPC and 10% of the water were withheld from the homogeniser, and a third where non-fat dry milk and 20% of the water were withheld from the homogeniser. They found that the WPC homogenised mix exhibited twice the fat destabilisation as when WPC was withheld and concluded that enhanced levels of whey proteins versus caseins at the interface during homogenisation led to emulsions that exhibited more fat destabilisation during freezing; the whey proteins reduced the interfacial tension further than the caseins, thus favouring their adsorption at the interface.
Goff et al. (1989) also noted that if milkfat is emulsified with a minimal amount of milk protein, then an emulsion that is quiescently stable but has a protein surface concentration low enough to undergo partial coalescence during freezing can be created without added emulsifier. Similarly, Mussellwhite & Walker (1971) note that an ice cream mix formulated without added emulsifier could be prepared in two phases: one, an emulsion containing all of the fat, a portion of the water and an appropriate fraction of the proteins. The other would be a serum containing the bulk of the protein as well as the other ingredients such as sweetnerens and stabilisers dissolved in the remaining water. These two phases would be pasteurised and homogenised separately and combined only just before freezing due to the danger of the emulsified droplets adsorbing additional protein from the serum and becoming too heavily coated (Segall & Goff, 1999).
References
Alderman R., and Hartel, R. W., 2001. Lipid crystallization and its effect on the physical structure of ice cream. In: Garti, N., Sato, K., (eds) Crystallization processes in fats and lipid systems. New York: MarcelDekker. pp 381–427
Darling, D. F., 1982. Recent advances in the destabilization of dairy emulsions. Journal of Dairy Research. 49(4). 695-712
Daw, E., and Hartel, R. W., 2015. Fat destabilization and melt-down of ice creams with increased protein content. International Dairy Journal. 43.33-41.
Dickinson, E., and Gelin, J. L. 1992. Influence of emulsifier on competitive adsorption of as-casein and b-lactoglobulin in oil-inwater emulsions. Colloids Surf. 63:329.
Dickinson, E., 2003. Interfacial, emulsifying, and foaming properties of milk proteins. In P.,. F. Fox & P. L. H. McSweeney (Eds), Advanced dairy chemistry: proteins part B (3rd ed, Bol. 1, pp. 1229-1260. New York: Springer.
Euston, S. E., Singh H., Munro, P. A., and Dalgleish, D. G., 1995. Competitive adsorption between sodium caseinate and oilsoluble and water-soluble surfactants in oil-in-water emulsions. Journal of Food Science. 60:1151.
Fredrick, E., Walstra, P., and Dewettinck, K., 2010. Factors governing partial coalescence in oil-in-water emulsions. Advances in Colloid and Interface Science. Volume 153(1)
Fuller, G. T., Considine, T., Golding, M., Matia-Merino, L., and MacGibbon, A., 2015. Aggregation behaviour of partially crystalline oil-in-water emulsions: Part II - Effect of solid fat content and interfacial film composition on quiescent and shear stability. Food Hydrocolloids. Volume 51.
Goff, H. D., Liboff, M., Jordan, W. K., and Kinsella, J.E., 1987. The effects of polysorbate 80 on the fat emulsion in ice cream mix: evidence from transmission electron microscopy studies. Food Microstruct. 6:193.
Goff, H. D., and Jordan, W. K., 1989. Action of emulsifiers in promoting fat destabilization during the manufacture of ice cream. Journal of Dairy Science. 72:18.
Goff, H. D., Kinsella, J. E., and Jordan W. K., 1989. Influence of Various Milk Protein Isolates on Ice Cream Emulsion Stability. Journal of Dairy Science. 72:385--397
Goff, H. D., 1997. Instability and Partial Coalescence in whippable Dairy Emulsions. Journal of Dairy Science. 80:2620-2630.
Goff, H. D., and Hartel R. W., 2013. Ice Cream. Seventh Edition. New York Springer.
Hinrichs J., and Kessler, H., 1997. Fat content of milk and cream and effects on fat globule stability. Journal of Food Science. 62: 992-5.
Muse, M. R., and Hartel, R. W., 2004. Ice Cream Structural Elements that Affect Melting Rate and Hardness. Journal of Dairy Science. 87:1-10
Musselwhite, P. R., and D. A. Walker. 1971. The effect of the colloidal state of the emulsion on ice cream structure. Journal of Texture Studies. 2:110.
Segall, K. I., and Goff, H. D., 1999. Influence of adsorbed milk protein type and surface concentration on the quiescent and shear stability of butteroil emulsions. International Dairy Journal. 9 683 -691
Walstra, P., 2003. Physical chemistry of foods. New York: Marcel Dekker.
Williams, A., and Dickinson, E., 1995. Shear-induced instability of oil-in-water emulsions. Page 252 in Food Macromolecules and Colloids. E. Dickinson and D. Lorient, ed. R. Soc. Chem., London, England.