Fiber in ice cream
 The use of fiber in ice cream increases its nutritional value, improves texture, prolongs shelf-life, and can replace between 25% and 50% of the fat. In this post, we will be looking at the use of wheat fiber, oat fiber, inulin, apple fiber, and orange peel fiber in ice cream production.
The use of fiber in ice cream increases its nutritional value, improves texture, prolongs shelf-life, and can replace between 25% and 50% of the fat. In this post, we will be looking at the use of wheat fiber, oat fiber, inulin, apple fiber, and orange peel fiber in ice cream production.
You might also like to read:
• Lello 4080 Musso Lussino Ice Cream Maker - A Comprehensive Review• Cuisinart ICE-100 Ice Cream Maker - A Comprehensive Review• Vanilla Bean Gelato - Recipe• The role of fat in ice cream• Why is corn syrup used in ice cream?[toc]
1. Nutritional value
Dietary fiber has been associated with a wide range of health benefits, including a reduced risk of coronary heart disease, postprandial blood glucose levels, type 2 diabetes, obesity, dyslipoproteinaemia, cardiovascular disease, and colon cancer; the lowering of cholesterol; the increase of calcium bioavailability; and the reinforcement of the immunological system ([^1] [^2] [^3] [^4]). To make dietary-fiber claims, fiber should be added to ice cream in amounts that range from 3g to 6g per 100 g or per 100 ml ([^5]).
2. Improve texture
Dietary fiber can be used in ice cream production to improve texture through its viscosity enhancement and freezing point depression.
2.1. Viscosity enhancement
Smooth and creamy texture, primarily associated with a high milk fat content and small ice crystal size, is also strongly influenced by the viscosity of the ice cream mix ([^6] [^7]). Viscosity can be loosely defined as the thickness of a liquid, with thicker liquids having higher viscosities (honey has a higher viscosity than water for example). In general, as the viscosity of an ice cream mix increases, the smoothness of texture, body, and resistance to melting increase, but the overrun (the air that is whipped into the ice cream) decreases ([^8]).
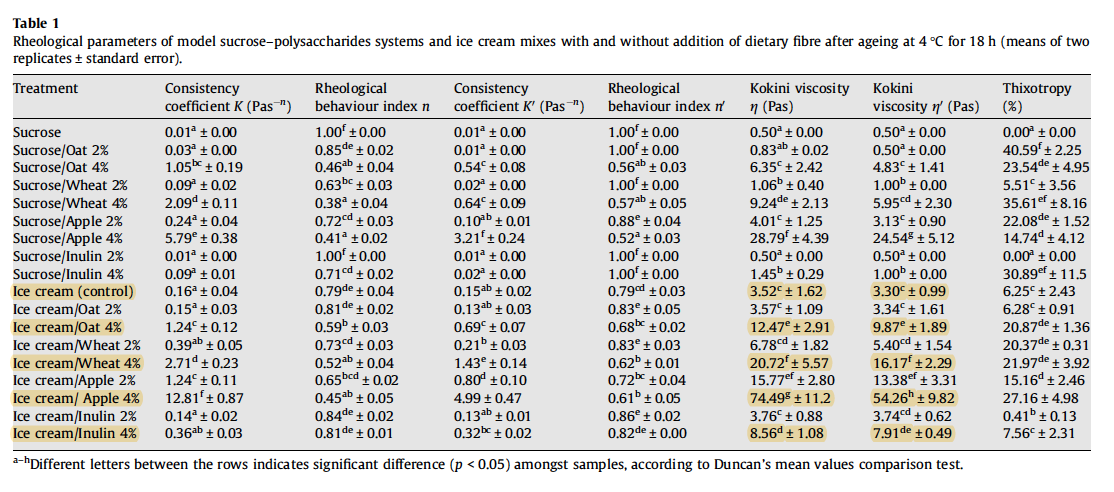
Soukoulis et al.([^9]) investigated the effects of four dietary fiber sources (oat, wheat, apple, and inulin) on the rheological and thermal properties of ice cream. The composition of the ice cream mix tested was 6% fat, 11% milk solids not fat, 16% sugar solids, 0.2% stabiliser, 0.2% emulsifier, and 2% or 4% dietary fiber. The researchers found that the addition of 4% fiber significantly enhanced viscosity, with apple fiber leading to the most pronounced increase. Pectin, which is naturally found in apples and is well known for its gel-forming ability, can explain the intense enhancement of viscosity, which was 3-15 times greater than the control (the sample without added fiber).
2.1.1. Effect of increased viscosity on ice crystal size
Smooth and creamy ice cream requires the majority of its ice crystals to be small. If many crystals are large, the ice cream will be perceived as being coarse or icy. As the viscosity of an ice cream mix increases, the smoothness of texture increases owing to a reduction in the average crystal size. This effect can be explained by two facts. First, the higher viscosity promotes crystal melting and attrition. Second, the mix has a higher resistance to water diffusion to the ice crystal surface, which reduces the growth rate ([^10]).
2.1.2. Effect of increased viscosity on meltdown rate
Ice cream is a system composed of four structural phases. The three main structural phases are air cells, ice crystals, and fat globules, which are dispersed in an unfrozen, high-viscosity aqueous phase with sugars, milk proteins, and unfrozen water, called serum ([^8]). An increase in the viscosity of the unfrozen serum phase will significantly slow the rate at which ice cream melts ([^11] [^12]). Studies have found that increasing inulin ([^12] [^13]) and citrus fiber ([^14]) concentrations decreases the ice cream melting rate.
2.1.3. Effect of increased viscosity on overrun
An increase in the viscosity of the serum phase enhances the stabilisation of air cells and allows them to be reduced to smaller sizes ([^15] [^16]). Smaller dispersed air cells produce a creamier mouthfeel during consumption ([^17]), and higher overrun values ([^18] [^19]). In turn, higher overrun values result in slower melting, since air cells act as an insulator medium ([^20] [^21] [^8] [^22])
An increased overrun in reduced-fat ice creams employing inulin was reported by Akalin and Erisir[^22], and by Akalin et al.[^23] . Dervisoglu & Yazici [^14], however, found that the use of citrus fiber led to the significant improvement of the melting quality of ice cream but failed to improve viscosity, overrun, and texture.
2.2. Freezing point depression
The freezing point of pure water is 0°C (32°F). When a substance is dissolved in water, however, the temperature at which the water freezes is lowered. This lowering of the freezing point is referred to as the ‘Freezing Point Depression’ and is defined as the difference between 0°C (32°F) and the temperature at which water in an ice cream mix first begins to freeze ([^24]).
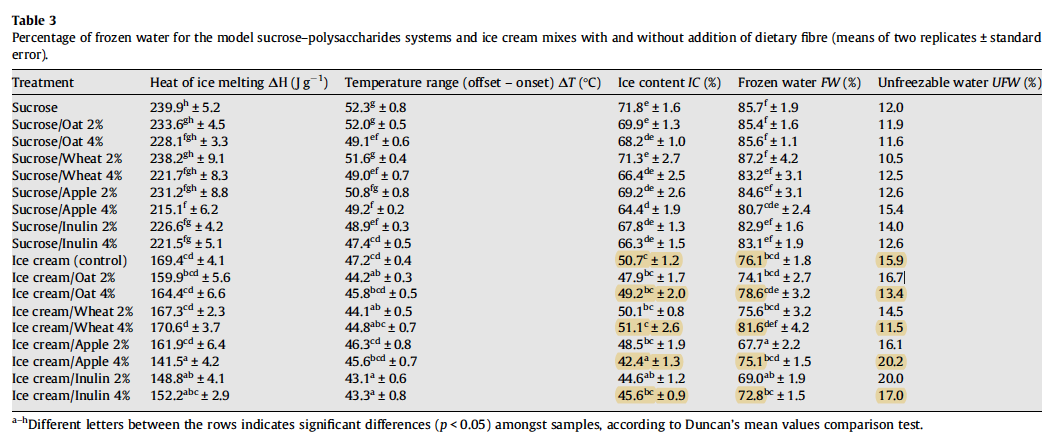
The extent of freezing point depression is a critical parameter in ice cream production as it influences the initial mean size of the formed ice crystals and also their native thermodynamic instability, which leads to their gradual growth during storage ([^25]). A lower freezing point results in a lower ice phase volume (less frozen water) and, therefore, smaller average ice crystal size ([^25] [^26]). Mellado([^27]) found that there was a substantial reduction of the average crystal size when the ice phase volume dropped from 53.5% to 43.9% and 39.5%.
Soukoulis et al.([^9]) found that oat and wheat fiber addition caused a significant decrease of the freezing point temperature. In contrast, apple fiber and inulin increased the freezing point temperature. Interestingly, the freezing point depression was found to be more pronounced in the 2% fiber-enriched formulations, as opposed to the 4% fiber-enriched formulations. The researchers, however, did not find a clear relationship between freezing point depression and the ice phase volume, i.e. a lower freezing point resulting in less frozen water. Interestingly, the ice phase volume was found to be primarily dependent on the bound (unfreezable) water and less on the freezing point, indicating that the water-binding capacity and the impact of ingredients on the steric inhibition of water molecules controlled the ice formation phenomena. Love and Haraldsson([^28]) discovered that smaller, more numerous ice crystals were found when a greater amount of water was bound. The retardation of the migration of free water encouraged nucleation (birth of new crystals) rather than the continued growth of existing ice crystals.
Soukoulis et al.([^9]) found that the addition of wheat or oat fiber led to significant increase in the percentage of frozen water. Contrariwise, the presence of apple fiber and inulin led to a significant decrease in the percentage of frozen water, which would suggest the formation of smaller ice crystals.
3. Prolong shelf-life
Dietary fiber can also be used in ice cream production to prolong shelf life through its ability to reduce ice crystal growth during storage.Not all of the water in ice cream is frozen. This water contains dissolved sugars and salts, as well as any aqueous phase proteins and stabilisers ([^24]). During storage, unfrozen water moves from the serum phase to the crystal surface and enhances ice crystal growth and recrystallisation ([^29] [^30]). Recrystallisation is defined as “any change in number, size, shape… of crystals” ([^31]) and basically involves small crystals disappearing, large crystals growing, and crystals fusing together. This increase in crystal size eventually reaches a point where the ice cream develops coarse texture, at which point it has surpassed its shelf life. Recrystallisation occurs mostly by two mechanisms: accretion and migration ([^32]). Accretion is the joining together of two or more adjacent ice crystals to form a single, larger crystal. Migration involves melting of smaller crystals and movement of the melted liquid to the surface of larger crystals. This mechanism is influenced by the temperature within the ice cream. At higher temperatures, the smaller ice crystals melt partially or completely and when the temperature is lowered again, the liquid refreezes on the larger crystals ([^32]). Migration is influenced greatly by the rate at which the water molecules diffuse to the larger ice crystal surface, which is known as diffusion kinetics. The movement, or diffusion, of the crystals is largely dependent on the viscosity of the serum phase. If the viscosity is high, the rate of diffusion, and therefore the rate of recrystallisation, is slow ([^33]).
3.1. The glass transition temperature
When ice cream is cooled below its glass transition temperature (typically below -32°C (-25.6°F)), due to the tremendous increase of viscosity, the unfrozen serum phase becomes glassy in nature, and limited molecular mobility means that recrystallisation occurs very slowly ([^34] [^35] [^36] [^37]).
Soukoulis et al.([^9]) found that the addition of dietary fiber led to a significant increase of the glass transition temperature. The researchers found that the addition of inulin had the strongest elevating effect on the glass transition temperature, and, thus, the strongest barrier against water diffusion and recrystallisation during storage. The addition of wheat and oat fiber led only to a slight elevation of the glass transition temperature. In the absence of proteins, apple fiber had only a slight effect on elevating the glass transition temperature. When protein was added, however, the elevation of the glass transition temperature was greater. Soukoulis et al.([^9]) found that the gelation of pectin contributed to the increase of the glass transition temperature, but also to the induction of phase separation, known as 'wheying off', due to the incompatibility of proteins with pectin. Wheying off refers to the leaking of a clear watery serum layer during the melting of ice cream, which has an undesirable appearance ([^24]).
4. Reduction of fat
Conventional ice cream formulations have a high fat content of between 10 to 18%, which can be replaced with fiber. Pintor et al.([^13]) found that it was possible to reduce up to 25% of the fat with 3% inulin, with good textural and sensory characteristics of the final product. The substitution of fat with inulin increased the ice cream mix viscosity, improved air incorporation, decreased the ice cream melting rate, and produced ice cream with soft and homogenous texture. Colour characteristics were not affected by the replacement. The researchers also showed that fat-reduced inulin ice cream was not perceived different to commercial vanilla ice cream.
Crizel et al.[^38] found that the use of 1.0% of pre-treated orange peel fiber reduced the ice cream fat content by 50%, and that its overall acceptance did not differ from that of the control ice cream. The ice cream supplemented with orange fiber, however, showed significantly lower overrun values compared with those of the control ice cream, likely due to the reduction in fat. The researchers found that when orange peel fiber that had not been pre-treated by hydro-distillation to reduce the levels of compounds responsible for bitterness was used, flavour and aftertaste scores were negatively affected. It was also found that the addition of orange fiber resulted in a yellow ice cream. A similar result was obtained by Dervisoglu & Yazici[^14] for ice cream supplemented with citrus fiber.
5. My tests
5.1. Control
My control sample consisted of 23% fat, 16% sugar, 4% egg yolks, 12% not fat-milk-solids, and 55% total solids. This was heated to 77°C (170°F), kept at this temperature for 30 minutes, cooled in an ice bath, aged overnight at 2°C (35°F), and frozen the next day in my Lello 4080 Musso Lussino. This produced ice cream that was extremely smooth, dense, and creamy.
5.2. Orange fiber
To attempt to replicate the findings by Crizel et al.[^38] that 1.0% of pre-treated orange peel fiber can be used to reduce the fat content by 50%, I prepared a sample with the same composition as the control, but with a reduced fat content of 11.5% (50% of the fat of my control sample) and 1% orange fiber. This sample was prepared and frozen in the same way as the control. In concurrence with the findings by Crizel et al.[^38], I found that 1% orange fiber can be used to reduce the fat content in ice cream by 50%: this produced extremely smooth, dense, and creamy ice cream that was comparable in texture to the control. I did find, however, that the addition of 1% orange fiber imparted a noticeably bitter aftertaste that made the ice cream unpalatable. To try to reduce this negative bitter aftertaste, I prepared a second sample with the same composition as the control, but reduced the orange fiber from 1% to 0.5% and increased the fat content to 17% (a roughly 25% decrease compared to the control). I found that this produced extremely smooth, dense, and creamy ice cream that was comparable in texture to the control, had a pleasant yellow colour, and just a very faint lingering fibrous taste that wasn't objectionable. Most importantly, reducing the orange fiber content to 0.5% removed the negative lingering bitterness. Two other tasters liked this sample and weren't able to detect the faint fibrous taste or any lingering bitterness.
5.3. Apple fiber
I prepared a third sample with the same composition as the control, but with a reduced fat content of 17% (a roughly 25% decrease compared to control sample) and 4% apple fiber. This sample was prepared and frozen in the same way as the control. I sadly didn't have much luck with this sample. Although the addition of 4% apple fiber led to the most significant increase of viscosity, I found that this sample produced ice cream that was extremely chewy, had objectionable fibrous specs that were detectable in the mouth, and a dark red colour. The addition of 4% apple fiber did, however, significantly increase melting resistance and impart a pleasant, sweet, apple flavour that was reminiscent of a sweet health/breakfast bar. I almost think that this ice cream could be marketed as a high-fiber dessert. I didn't get the phase separation reported by Soukoulis et al.([^9]). 
5.4. Inulin
I prepared a fifth sample with the same composition as the control, but with a reduced fat content of 17% (a roughly 25% decrease compared to the control) and 4% inulin. This sample was prepared and frozen in the same way as the control. I found that this inulin sample produced the most favourable results: it produced extremely smooth and creamy ice cream that was comparable in texture and colour to the control. Furthermore, the addition of 4% inulin did not adversely affect flavour. Contrary to the findings reported by Soukoulis et al.([^9]), El-Nagar et al.[^12] and Pintor et al.[^13], I found that the addition of 4% inulin depressed the freezing point, producing ice cream that was softer and easier to scoop straight out of the freezer, but melted quicker than the control. It is likely, however, that the reduction of the fat content by roughly 25% contributed to the increased melting rate as ice cream containing a high amount of fat tends to melt more slowly ([^39] [^40] [^41]).
5.5. Wheat and oat
I wasn't abel to source wheat and oat fiber for my tests. I may try to get my hands on some oat fiber in the coming weeks to update this post.
5. Summary
Fiber (apple, inulin, wheat, oat, and orange peel) can be used in ice cream production at either 4% (apple, inulin, wheat, and oat) or 1% (orange peel fiber) to increase its nutritional value, improve texture, increase overrun, reduce the rate at which ice cream melts, and to reduce ice crystal growth during storage, thus extending shelf-life.
It is also possible to reduce 25% of the fat in an ice cream mix with 4% inulin, or 50% fat with 1% pre-treated orange peel fiber, although the latter does impart an objectionable lingering bitterness. Reducing orange fiber from 1% to 0.5% removes this objectionable lingering bitterness and allows for the reduction of 25% of the fat.
In my tests, I didn't have much luck with 4% apple fiber: this sample produced ice cream that was extremely chewy, had objectionable fibrous specs that were detectable in the mouth, and a dark red colour. I found that the addition of 4% inulin had the most favourable results, producing extremely smooth and creamy ice cream that had 25% less fat, was softer and easier to scoop, and had a pleasant flavour and colour profile. Contrary to other studies, however, I found that the addition of 4% inulin increased the rate at which the ice cream melted, although the reduction of fat also likely contributed to the increased melting rate.
I've posted two recipes containing inulin: my Vanilla Bean Gelato Recipe and my Egg-Free Vanilla Bean Ice Cream Recipe.
6. References
[^1]: Thebaudin, J. Y., Lefebvre, A. C., Harrington, M., and Bourgeois, C. M., 1997. Dietary fibers: Nutritional and technological interest. Trends in Food Science and Technology. 8, 41–49.[^2]: Tungland, B. C., and Meyer, D., 2002. Nondigestible oligo and polysaccharides (dietary fiber): Their physiology and role in human health and food. Comprehensive Reviews in Food Science and Food Safety. 1, 73–92.[^3]: EFSA., 2010. Scientific opinion on dietary reference values for carbohydrates and dietary fiber. EFSA Journal. 8(3), 1462.[^4]: Hauner, H., Bechthold, A., Boeing, H., Brönstrup, A., Buyken, A., Leschik-Bonnet, E., et al., 2012. Evidence-based guideline of the German nutrition society: Carbohydrate intake and prevention of nutrition-related diseases. Annals of Nutrition & Metabolism, 60(Suppl. 1), 1–58.[^5]: European Commission., 2008. Amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowances, energy conversion factors and definitions. Annex II. Official Journal of the European Union, L285, 9–12.[^6]: Mela, D. J., Langley, K. R., and Martin, A., 1994. Sensory assessment of fat content: effect of emulsion and subject characteristics. Appetite, 22, 67–81.[^7]: Akhtar, M., Stenzel, J., Murray, B. S., and Dickinson, E., 2005. Factors affecting the perception of creaminess of oil-in-water emulsions. Food Hydrocolloids, 19. 521-526.[^8]: Marshall, R. T., Goff, H. D., and Hartel R. W., 2003. Ice cream (6th ed). New York: Kluwer Academic/Plenum Publishers.[^9]: Soukoulis, C., Lebesi, D., and Tzia, C., 2009. Enrichment of ice cream with dietary fiber: Effects on rheological properties, ice crystallisation and glass transition phenomena. Food Chem. 115:665–671.[^10]: Amamou, A. H., Benkhelifa, H., Alvarez, G., and Flick, D., 2010. Study of crystal size evolution by focused-beam reflectance measurement during the freezing of sucrose/water solutions in a scraped-surface heat exchanger. Process Biochemistry. 45.1821-1825.[^11]: Muse, M. R., and Hartel, R. W., 2004. Ice Cream Structural Elements that Affect Melting Rate and Hardness. Journal of Dairy Science. 87, 1-10.[^12]: El-Nagar, G., Clowes, G., Tudorica, C. M., Kuri, V., and Brennan, C. S., 2002. Rheological quality and stability of yog-ice cream with added inulin. Int J Dairy Technol. 55(2):89–93.[^13]: Pintor, A., Severiano-Perez, P., and Totosaus, A., 2013. Optimization of fat-reduced ice cream formulation employing inulin as fat replacer via response surface methodology. Food Science and Technology International. 20(7). 489-500.
[^14]: Dervisoglu, M., and Yazici, F., 2006. The effect of citrus fiber on the physical, chemical, and sensory properties of ice cream. Food Sci Tech Int. 12(2):159-164.
[^15]: Hirt, D. E., Prud’homme, R. K., and Rebenfeld, L., 1987. Characterization of foam cell size and foam quality using factorial design analyses. Journal of Dispersion Science and Technology. Volume 8.[^16]: Den Engelsen, C. W., Isarin, J. C., Gooijer, H., Warmoeskerken, M. M. C. G., and Groot Wassink, J., 2002. Bubble size distribution of foam. AUTEX Research Journal. Volume 2(1).[^17]: Eisner, M. D., Wildmoser, H., and Windhab, E, J., 2005. Air cell microstructuring in a high viscous ice cream matrix. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 263(1).
[^18]: Chang, Y., and Hartel, R. W., 2002. Development of air cells in a batch ice cream freezer. Journal of Food Engineering. 55, 71-78.
[^19]: Sofjan, R. P., and Hartel, R. W., 2003. Effects of overrun on structural and physical characteristics of ice cream. International Dairy Journal. 14.255-262.[^20]: Sakurai, K. S., Kokubo, K., Hakamata, M., and, Yoshida, S., 1996. Effect of production conditions on ice cream melting resistance and hardness. Milchwissenschaft. 51: 451–454.[^21]: Caillet, A., Conge, C., Andrieu, J., Laurent, P., and Rivoire, A., 2003. Characterization of ice cream structure by direct optical microscopy. Influence of freezing parameters. LWT-Food Science and Technology. 36: 743–749.[^22]: Akalin, A. S., and Erisir, D., 2008. Effects of inulin and oligofructose on the rheological characteristics and probiotic culture survival in low-fat probiotic ice cream. J. Food Sci. 73:M184-M188.[^23]: Akalin, A. S., Karagozlu, C., and Unal, G., 2008. Rheological properties of reduced-fat and low-fat ice cream containing whey protein isolate and inulin. European Food Research and Technology. 227: 889–895.[^24]: Goff, H. D., and Hartel R. W., 2013. Ice Cream. Seventh Edition. New York: Springer.[^25]: Hartel, R. W., 2001. Crystallization in foods (1st ed.). Gaithersburg, Maryland: Aspen Publishers Inc..[^26]: Sutton, R. L., and Wilcox, J., 1998. Recrystallization in model ice cream solutions as affected by stabilizer concentration. Journal of Food Science. 63. 1.[^27]: Mellado, A. A. F., 1998. Ice crystallization and recrysatllization in frozen model solutions and ice cream as affected by polysaccharide gums. A thesis presented to the Faculty of Graduate Studies of the University of Guelph.[^28]: Love, R. M., and Haraldsson, S. B., 1961. Ice crystal formation and cell damage in cod muscle frozen before rigor mortis. J. Sci. Food Agric. 12: 442.[^29]: Donhowe, D. P., and Hartel, R. W., 1996. Recrystallization of ice in ice cream during controlled accelerated storage. Int Dairy J, 6 (11–12):1191–208.[^30]: Hartel, R. W., 1998. Phase transitions in ice cream. In: RaoMA, Hartel RW, editors. Phase/state transitions in foods: chemical, structural, and rheological changes. IFT basic symposium series. New York: Marcel Dekker. p 327–68.[^31]: Fennema, O. R., Powrie, W. D., Marth, E. H., 1973. Low Temperature Preservation of Foods and living Matter. USA: Marcel Dekker, Inc.[^32]: Sutton, R., and Bracey, J., 1996. The blast factor. Dairy Industries International. 61(2):31-33.[^33]: Schmidt, K., Herald, T., and Flores, R. A., 2000. Mechanisms of ice crystallization and recrystallization in ice cream: A review. Food Reviews International. 16(3). 259-271.
[^34]: Herrera, M. L., M’Cann, J. I., Ferrero, C., Zaritzky, N. E., and Hartel, R. W., 2007. Thermal, mechanical and molecular relaxation properties of frozen sucrose and fructose solutions containing hydrocolloids. Food Biophysics. 2, 20–28.
[^35]: Kasapis, S., 2006. Glass transition in frozen foods and biomaterials. In Da Wen Sun (Ed.), Handbook of frozen food processing and packaging (pp. 33–51). New York: Taylor and Francis Group LCC.
[^36]: Regand, A., and Goff, H. D., 2003. Structure and ice recrystallization in frozen stabilized ice cream model systems. Food Hydrocolloids. 17, 95–102.
[^37]: Slade, L., and Levine, H., 1991. Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. In F. M. Clydesdale (Ed.), Critical reviews in food science and nutrition (pp. 155–360). Boca Raton: CRC Press.
[^38]: Crizel, T. M., Araujo, R. R., Rios, A. O., Rech, R., and Flores, S. H., 2014. Orange fiber as a novel fat replacer in lemon ice cream. Food Sci. Technol, Campinas. 34(2): 332-340.
[^39]: Roland, A. M., Philips, L. G., and Boor, K. J., 1999. Effects of fat content on the sensory properties, melting, color, and hardness of ice cream. Journal of Dairy Science. 82, 32-38
[^40]: Alamprese, C., Foschino, R., Rossi, M., Pompei, C., and Savani, L., 2002. Survival of lactobacillus johnsonii la1 and influence of its addition in retail-manufactured ice cream produced with different sugar and fat concentrations. International Dairy Journal. 2, 201-208.
[^41]: Hyvonen, L., Linna, M., Tutorial, H., and Dijksterhuis, G., 2003. Perception of Melting and Flavor Release of Ice Cream Containing Different Types and Contents of Fat. Journal of Dairy Science. 86, 1130-1138.