How long does homemade ice cream last in the freezer?
14 MINUTE READThe shelf life of food is dependent on changes in the microbial content, chemical nature (e.g., flavour), and the physical attributes of the product (Goff & Hartel, 2013). In ice cream, microbial growth does not occur to any significant extent during storage of either the mix or of the frozen product, so that physicochemical changes are generally considered the most important in determining shelf life (Goff & Hartel, 2013).
In this post, I'll discuss the physicochemical changes in ice cream that affect shelf life. These will include: 1. ice recrystallisation; 2. lactose crystallisation; 3. a change in the size of air cells; and 4. changes in ice cream volume, leading to a defect known as shrinkage. I'll offer tips for extending shelf life and then summarise the physiochemical changes at the end.
YOU MIGHT ALSO FIND THE FOLLOWING POSTS USEFUL:
- Vanilla ice cream - Recipe
- Ice crystal formation and growth in ice cream
- Why does ice cream melt?
- Air bubbles in ice cream
1. ICE RECRYSTALLISATION
Ice crystal size is a critical factor in the development of smooth and creamy ice cream (Donhowe et al. 1991). Smooth and creamy ice cream requires the majority of ice crystals to be small, around 10 to 20 µm in size. If many crystals are larger than this, the ice cream will be perceived as being coarse or icy (Drewett & Hartel, 2007; Goff & Hartel, 2013).
Ice cream is frozen in two stages: dynamic and static freezing. Ice crystals are formed during dynamic freezing, where the ice cream mix is frozen and agitated in an ice cream machine to incorporate air, and grow during static freezing, where the partially frozen ice cream mix is hardened in a freezer without agitation. The primary aim is to promote the formation of as many small ice crystals as possible during dynamic freezing and then preserve these small crystals during static freezing and storage.
Ice recrystallisation (the general increase in ice crystal size) during storage is considered to have the most significant effect on the shelf life of ice cream because of the adverse effect on texture.
1.1. FACTORS AFFECTING ICE RECRYSTALLISATION
Numerous factors influence the rate of recrystallisation, including the freezing and hardening processes, storage conditions, and ice cream composition (Hartel, 1998).
1.1.1. DYNAMIC FREEZING
Nucleation (the birth of ice crystals) occurs only during dynamic freezing where the temperature at the cold freezer bowl wall is cold enough to form new crystals. The formation of smaller ice crystals and narrower initial ice crystal distribution during dynamic freezing generally lead to longer shelf life since the ice crystals must grow to a larger extent (Goff & Hartel, 2013). To read my discussion of the factors influencing ice crystal formation and growth during the freezing of ice cream, please click here.
1.1.2. STATIC FREEZING
No new ice crystals are formed during static freezing but the existing small crystals grow in size until the temperature decreases to -18°C (0.4°F), or ideally -25°C to -30°C (-9.4 to -20.2°F), to halt this growth. During static freezing, ice crystals typically grow by about 30% to 40% (Marshall et al., 2003) to an average size of about 25 to 45 µm (Berger et al., 1972; Donhowe & Hartel, 1996; Hagiwara & Hartel, 1996; Koxholt et al., 2000; Sofjan & Hartel, 2004). A mean ice crystal size of about 50 um is considered an average point where people start to notice coarse texture (Goff & Hartel, 2013).
Because higher temperatures accelerate recrystallisation, quick hardening to temperatures at or below -18°C (0.4°F) limits ice crystal growth (Donhowe, 1993; Hartel, 1996).
TIP#1 – QUICK HARDENINGTo promote faster hardening of the partially frozen ice cream during static freezing, place your ice cream at the back of your freezer where it’s usually the coldest. Efficient air flow in your freezer will also contribute to a reduction in the hardening time. Generally, the more compact the items in your freezer, the less efficient the air flow. The size of your batch also affects the cooling time, with larger batches usually taking longer to harden.
1.1.3. STORAGE CONDITIONS
Recrystallisation is also dependent on storage conditions, namely temperature and temperature fluctuations (Donhowe & Hartel, 1996), with colder storage temperatures better for extended shelf life.
1.1.3.1. STORAGE TEMPERATURE
The development of coarseness in ice cream during storage is highly temperature dependent, being particularly rapid at storage temperatures above about -14°C (6.8°F) (Donhowe & Hartel, 1996; Ben-Yoseph & Hartel, 1998). Earl & Tracy (1960) found that ice cream stored at -26.1°C (-14.98°F) suffered only slight textural deterioration after 16 weeks, but storage at -13.3°C (8.06°F) resulted in a coarse texture after only 2 weeks.
The ideal storage temperature to reduce recrystallisation would be below the glass transition temperature, around -32°C (-25°F), where recrystallisation occurs very slowly (Goff & Sahagian, 1996; Roos, 2010; Goff & Hartel, 2013). Goff & Hartel (2013) note that storage below about -25°C (-13°F) gives a sufficiently slow recrystallisation rate to give extended shelf life. Donhowe (1993) found that storage at -20°C (-4°F) resulted in very little increase in mean ice crystal size, but, at -5°C (23°F), mean ice crystal size increased from 40 µm to about 220 µm during 5 days of storage.
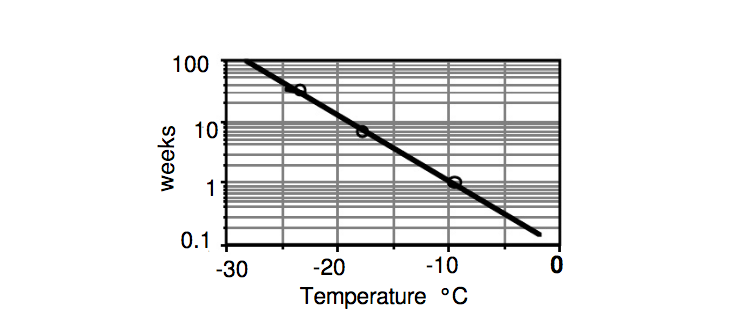
Labuza and Fu (1998) show shelf life data for ice cream based on sensory iciness perception. In their data, ice cream stored at -10°C (14°F) had a shelf life of about 1 week, whereas storage at -20°C (-4°F) and -25°C (-13°F) gave a shelf life of about 10 and 40 weeks respectively.
TIP #2 - LOWER THE STORAGE TEMPERATURETo reduce recrystallisation during storage and extend shelf life, store your ice cream at around -25°C (-13°F) or lower, ideally around -32°C (-25°F).
1.1.3.2. TEMPERATURE FLUCTUATIONS
Recrystallisation during storage is significantly slower for ice cream stored at a constant temperature than for ice cream stored at oscillating temperature conditions (Donhowe & Hartel, 1996). Witting & Smith (1986) showed that ice creams stored in a ‘supermarket-type frost/defrost freezer’ with temperature cycles between -9.4°C and -15°C (15.08°F and 5°F) became detectably icy in 4 weeks and objectionably icy in 3-10 weeks.
Temperature fluctuations may be associated with 1. changes in the storage temperature as the ice cream moves from point to point (e.g. from the supermarket to your freezer); 2. heat shocks, where the ice cream is left at room temperature for extended periods of time and then re-frozen; 3. temperature fluctuations due to automated defrost cycles; and 4. opening and closing of doors in freezers and storage cabinets (Goff & Hartel, 2013).
TIP #3 – MINIMISE TEMPERATURE FLUCTUATIONSTo minimise recrystallisation during storage, try to minimise temperature fluctuations by reducing the number of times you take your ice cream out of the freezer, leave it out at room temperature, and then re-freeze it. Also, try to limit the time that your tub of ice cream is left at room temperature by returning it to the freezer as soon as you’ve finished scooping. If possible, disable the auto defrost setting on your freezer and limit the number of times you open and close your freezer door.
1.1.4. COMPOSITIONAL FACTORS
Besides freezing and storage conditions, the composition of the ice cream mix influences recrystallisation. This includes the fat, protein, sweetener, and, stabiliser contents.
1.1.4.1. MILK FAT
Milk fat has been shown to influence development of coarseness during storage by providing a mechanical obstruction to ice crystal growth, slowing the rate of recrystallisation (Donhowe & Hartel, 1996; Prindiville et al., 1999). Goff & Hartel (2013) note that the tendency for ice crystals to grow decreases in the following order: nonfat, low-fat, light, reduced fat, regular, premium, and super premium ice creams.
I've found a butterfat content of around 23% to be optimum in homemade ice cream. Please click here to see my mix composition for my vanilla ice cream recipe.
1.1.4.2. MILK PROTEINS
Milk proteins play a role in reducing rates of ice recrystallisation, possibly through their water holding capacity (Regend & Goff, 2002, 2003).
1.1.4.3. SWEETENERS
Sweeteners affect recrystallisation by depressing the freezing point, or lowering the temperature at which the water in an ice cream mix starts to freeze (Harper & Shoemaker, 1983; Wittinger & Smith, 1986). For an ice cream mix with a lower freezing point, less water is frozen at a given temperature. Hagiwara & Hartel (1996) found that the lower the freezing point, or the larger the amount of unfrozen water in ice cream, the higher was the recrystallisation rate. Harper & Shoemaker (1983) found similar effects of freezing point for the effect of sweeteners on recrystallisation rates. These results suggest that ice cream formulations with the highest possible freezing point and the least amount of unfrozen water should have the greatest resistance to recrystallisation.
1.1.4.4. STABILISERS
Stabilisers are added to ice cream specifically to control ice recrystallisation (Bahramparvar & Tehrani, 2011). Sutton & Wilcox (1998) showed that both locust bean gum and guar reduced the extent of recrystallisation in ice cream compared with unstabilised ice cream. The inhibition of recrystallisation by these stabilisers was concentration dependent up to a level of about 0.3% after which further addition did not result in further inhibition. Sutton & Wilcox (1998) confirmed findings by Wittinger & Smith (1986) that locust bean gum was a better recrystallisation inhibitor than guar. Goff et al. (1993) also found that the ice crystal sizes before and after storage were smaller in stabilised ice cream than in ice cream without stabilisers.
1.1.4.5. ICE-STRUCTURING PROTEINS AND PROPYLENE GLYCOL MONOSTEARATE
Ice-structuring proteins (ISP), found primarily in cold-weather-adapted fish, insects, over-wintering plants, and in bacteria and fungi (Griffith & Ewart, 1995) have been found to reduce the recrystallisation rate. Regand & Goff (2006) studied ice recrystallisation in frozen sucrose solutions and in ice cream with or without ice structuring proteins from cold-acclimated winter wheat grass extract (AWWE). Significant ISP activity in retarding ice crystal growth was observed in all sucrose solutions containing 0.13% total protein from AWWE. Furthermore, in heat-shocked ice cream, ice recrystallisation rates during storage were significantly reduced with the addition of 0.0025 and 0.0037% total protein from AWWE. A remarkably smoother texture for ice creams containing ISP after heat-shock was also evident by sensory evaluation.
Propylene glycol monostearate (PGMS), an emulsifier used in cake mixes and aerated toppings, has also been identified as an inhibitor of ice recrystallisation at levels up to 0.5% (Barfod et al, 2005). The addition of 0.3% PGMS was found to decrease ice crystal size in ice cream both before and after heat shock (Aleong et al. 2008).
2. LACTOSE CRYSTALLISATION
Lactose crystallisation during storage can also result in a sandy or coarse texture. Lactose crystals can be detectable in the mouth when they exceed about 15 µm in size, compared to about 50 µm for ice crystals (Goff & Hartel, 2013). Lactose crystallisation occurs most readily when ice cream contains milk solids-not-fat (the lactose, proteins, minerals, water-soluble vitamins, enzymes, and some minor constituents) levels over about 16% and is stored at temperatures from -10°C to -15°C (14°F to 5°F) (Livney et al., 1995). Minimising storage time and temperature fluctuations, promoting the formation and stabilisation of many small air cells, adding stabilisers, and maintaining low storage temperatures help minimise lactose crystallisation.
3. AIR CELL SIZE
During dynamic freezing, air is incorporated into the mix through the folding and mixing action of the rotating dasher and scraper blades. Careful control of the amount of air incorporated into ice cream, or overrun, and the air cell size distribution is critical for ice cream texture, meltdown, and hardness (Sofjan & Hartel, 2003; Xinyi et al., 2010), with smaller dispersed air cells producing a creamier mouthfeel during consumption (Eisner et al., 2005).
Similar to ice crystals, mean air bubble size tends to increase over time in storage, with a larger rate of growth observed at warmer temperatures (Goff & Hartel, 2013). Pinzer et al. (2012) found that mean air bubble size increased rapidly at -5°C (23°F) and much more slowly at -15°C (5°F). This change in air bubble size contributes to a change in texture and quality of ice cream by enhancing crumbliness in body and significantly increasing the rate of meltdown.
4. SHRINKAGE
Another problem that occurs during the storage of ice cream is shrinkage, which appears as the ice cream pulling away from the walls of the container. Shrinkage occurs as the result of air bubbles coalescing (coming together) and forming continuous channels, which then leads to the collapse of the ice cream into these channels (Turan et al. 1999). Contributing factors can be high overrun, low solids, low protein, and changes in external pressure (Dubey & White, 1997). Goff & Hartel (2013) note that shrinkage occurs most often after ice cream experiences a significant decrease in pressure, as when ice cream is shipped across mountains or transported by plane, which first causes a volume expansion.
5. SUMMARY
Physicochemical changes in ice cream are generally considered the most important in determining shelf life. These include ice recrystallisation, lactose crystallisation, a change in the size of air cells, and changes in ice cream volume. Ice recrystallisation (the general increase in ice crystal size) during storage is considered to have the most significant effect on the shelf life of ice cream because of the adverse effect on texture.
The formation of smaller ice crystals during dynamic freezing, quick hardening to temperatures at or below -18°C (0.4°F) during static freezing, a low storage temperature of -25°C (-13°F) or lower, ideally around -32°C (-25°F), and minimising temperature fluctuations during storage help reduce recrystallisation and extend shelf life.
Based on sensory iciness perception, ice cream stored at -10°C (14°F) has a shelf life of about 1 week, whereas storage at -20°C (-4°F) and -25°C (-13°F) give a shelf life of about 10 and 40 weeks respectively.
I hope this post helps. I'd be happy to answer any questions so do get in touch and say hi! Ruben :)
References:
Aleong, J., Frochot, S., and Goff, H. D., 2008. Ice recrystallization inhibition in ice cream by propylene glycol monostearate. J Food Sci. 73(9).
Bahramparvar, M., and Tehrani, M. M., 2011. Application and function of stabilizers in ice cream. Food Rev Int. 27.
Barfod, N. M., da Lio, M., and Christensen, F. H., 2005. Process for the production of a frozen food product. International Patent WO/2005/060763
Ben-Yoseph E., and Hartel, R. W., 1998. Computer simulation of ice recrystallization in ice cream during storage. J Food Eng. 38.
Berger, K. G,, Bullimore, B. K., White, G. W., & Wright, W. B., (1972). The structure of ice cream – Part 1. Dairy Industries, 37(8).
Donhowe, D. P., 1993. Ice recrystallization in ice cream and ice milk. Ph.D. thesis, Univ. of Wisconsin-Madison, Madison.
Donhowe, D. P., Hartel R. W., and Bradley R.L., 1991. Determination of ice crystal size distributions in frozen desserts. Journal of Dairy Science. 74.
Donhowe, D. P., and Hartel, R. W., 1996. Recrystallization of ice in ice cream during controlled accelerated storage.Int. Dairy J. 6.
Drewett, E. M., and Hartel, R. W., 2007. Ice crystallisation in a scraped surface freezer. Journal of Food Engineering78(3).
Dubey, U. K., and White, C. H., 1997. Ice cream shrinkage. J Dairy Sci. 80.
Earl, F. A., and Tracey, P. H., 1960. The importance of temperature in the storage of ice cream. Ice Cream Trade J., 56(11).
Eisner, M. D., Wildmoser, H., and Windhab, E. J., 2005 Air cell microstructuring in a high viscous ice cream matrix. Coll Surf A: Physicochem Eng Aspects. 263.
Goff, H.D., Caldwell, K. B., Stanley, W., and Maurice, T. J., 1993. The influence of polysaccharides on the glass transition in frozen sucrose solutions and ice cream. J. Dairy Sci. 76.
Goff, H. D., and Sahagian, M. E., 1996 Glass transitions in aqueous carbohydrate solutions and their relevance to frozen food stability. Thermochim Acta. 280.
Goff, H. D., and Hartel R. W., 2013. Ice Cream. Seventh Edition. New York Springer.
Griffith, M., and Ewart, K. V., 1995. Antifreeze proteins and their potential use in frozen foods. Biotechnol. Adv. 13:375–402.
Hagiwara, T., and Hartel, R. W., 1996. Effect of sweetener, stabilizer and storage temperature on ice recrystallization in ice cream. J. Dairy Sci. 79:735–744.
Harper, E. K., and Shoemaker, C. F., 1983. Effect of locust beam gum and selected sweetening agents on ice recrystallization rates. Journal of Food Science. 48:1801.
Hartel, R. W., 1996. Ice crystallisation during the manufacture of ice cream. Trends in Food Science & Technology. 7(10).
Hartel, R. W., 1998. Mechanisms and kinetics of recrystallization in ice cream. In: Reid DS (ed) The properties of water in foods: ISOPOW 6. Blackie, London.
Koxholt, M., Eisenmann, B., and Hinrichs, J., 2000. Effect of process parameters on the structure of ice cream. Eur Dairy Mag. 1, 27-30.
Labuza, T. P., and Fu, B., 1997. Shelf life testing: Procedures and prediction methods. In: Erickson MC, Hong YC (eds) Frozen Food Quality.
Livney, Y. D., Donhowe, D. P., and Hartel, R. W., 1995. Influence of temperature on crystallization of lactose in ice cream. Int J Food Sci Technol. 30.
Marshall, R. T., Goff, H. D., and Hartel R. W., 2003. Ice cream. 6th ed. New York: Kluwer Academic/Plenum Publishers.
Pinzer, B. R., Medebach, A., Limbach, H. J., Dubois, C., Stampanoni, M., and Schneebeli, M. 2012 3D-characterization of three-phase systems using X-ray tomography: tracking the microstructural evolution in ice cream. Soft Matter. 8.
Prindiville, E. A., Marshall, R. T., Heymann, H. 1999. Effect of milk fat on the sensory properties of chocolate ice cream. J Dairy Sci. 82.
Regand, A., and Goff, H. D., 2002. Effect of biopolymers on structure and ice recrystallization in dynamically-frozen ice cream model systems. J Dairy Sci.
Regand, A., and Goff, H. D., 2003. Structure and ice recrystallization in frozen stabilized ice cream model systems. Food Hydrocoll. 17.
Regand, A., and Goff, H. D., 2006. Ice recrystallization inhibition of ice structuring proteins from winter wheat grass in model solutions and ice cream. J Dairy Sci. 89.
Roos, Y. R., 2010. Glass transition temperature its relevance in food processing. Annu Rev Food Sci Technol. 1.
Sutton, R. L., and Wilcox, J., 1998. Recrystallisation in ice cream as affected by stabilisers. Journal of Food Science. 63(1).
Sofjan, R. P., and Hartel, R. W., 2003. Effects of overrun on structural and physical characteristics of ice cream. International Dairy Journal. 14.
Turan, S., Kirkland, M., Trusty, P. A., and Campbell, I., 1999. Interaction of fat and air in ice cream. Dairy Industries Int. 64.
Wittinger, S. A., and Smith, D. E., 1986. Effect of sweeteners and stabilizers on selected sensory attributes and shelf life of ice cream. J Food Sci. 51(6).
Xinyi, E., Pei, Z. J., and Schmidt, K. A., 2010. Ice cream: foam formation and stabilization—a review. Food Rev Int. 26.